Summary information and primary citation
- PDB-id
- 8jbb; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase
- Method
- X-ray (1.81 Å)
- Summary
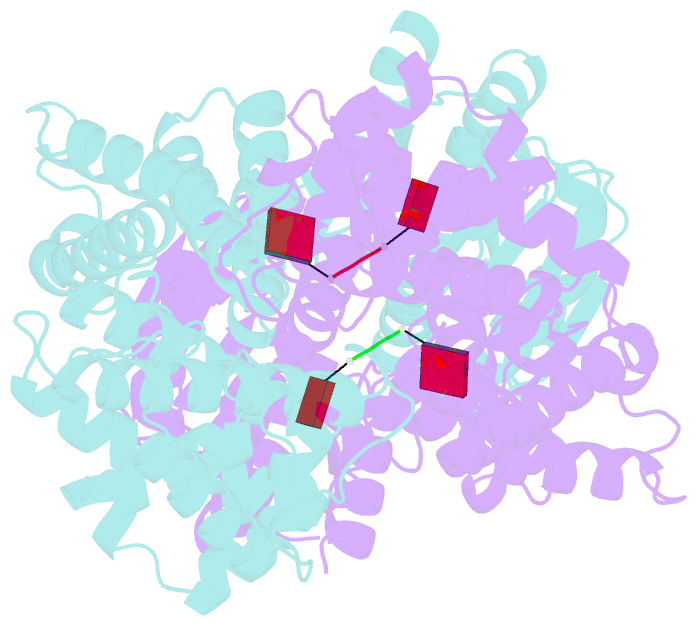
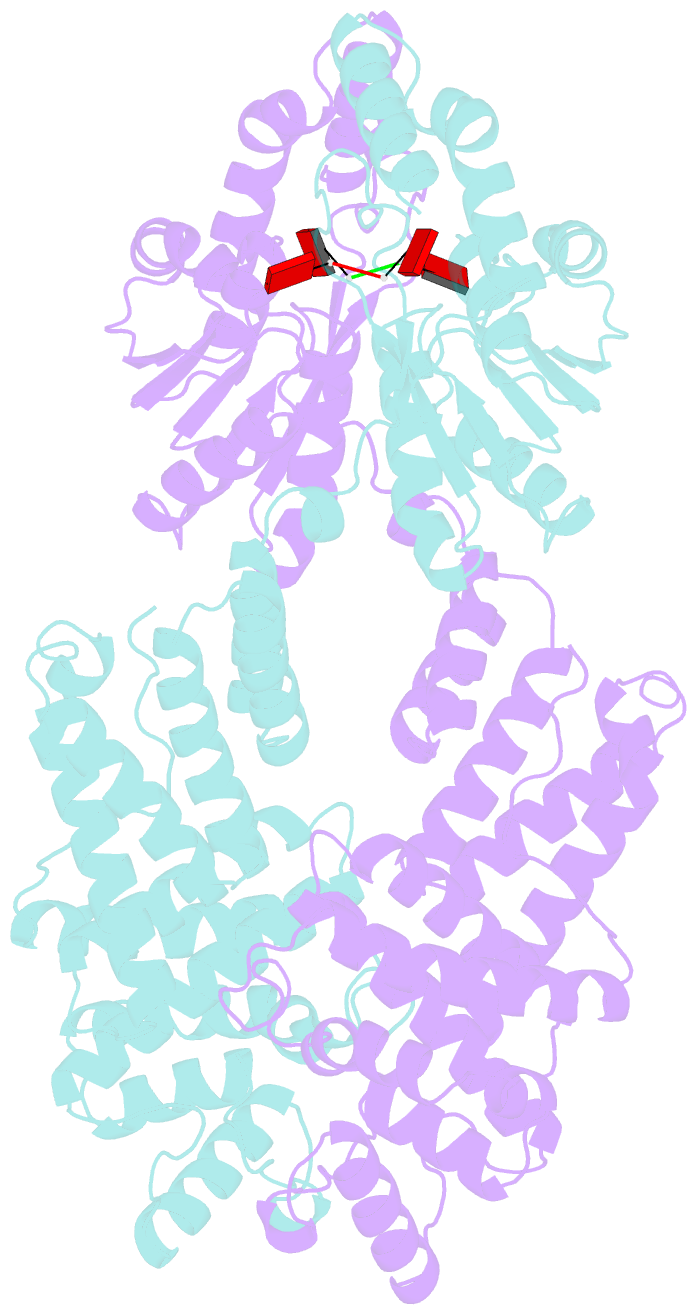
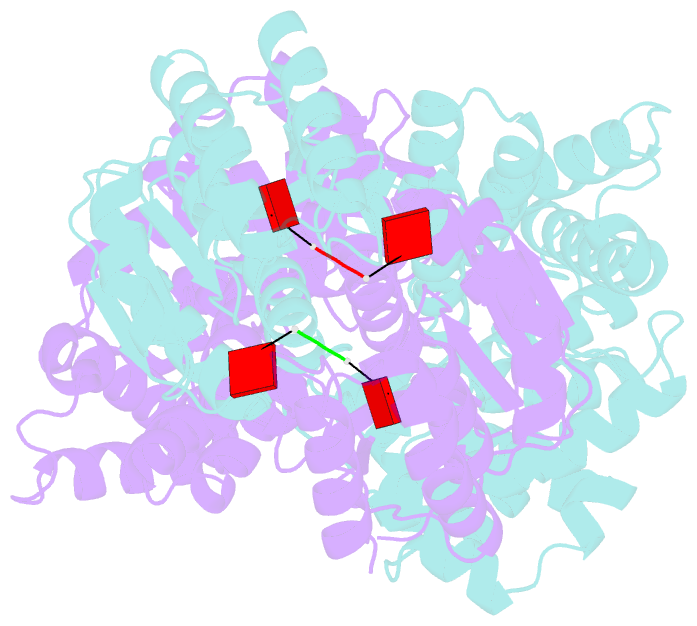
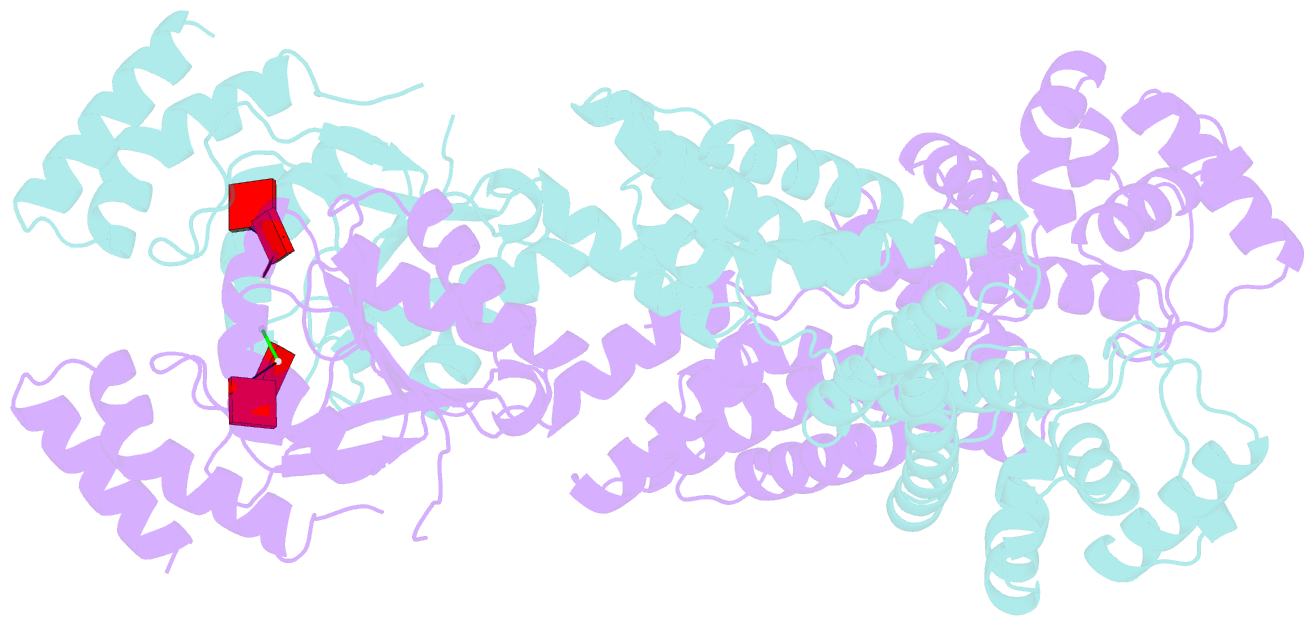
- Crystal structure of the csm6 from thermus thermophilus hb8 in complex with a2>p
- Reference
- Du L, Zhu Q, Lin Z (2024): "Molecular mechanism of allosteric activation of the CRISPR ribonuclease Csm6 by cyclic tetra-adenylate." Embo J., 43, 304-315. doi: 10.1038/s44318-023-00017-w.
- Abstract
- Type III CRISPR systems are innate immune systems found in bacteria and archaea, which produce cyclic oligoadenylate (cOA) second messengers in response to viral infections. In these systems, Csm6 proteins serve as ancillary nucleases that degrade single-stranded RNA (ssRNA) upon activation by cOA. In addition, Csm6 proteins also possess cOA-degrading activity as an intrinsic off-switch to avoid degradation of host RNA and DNA that would eventually lead to cell dormancy or cell death. Here, we present the crystal structures of Thermus thermophilus (Tt) Csm6 alone, and in complex with cyclic tetra-adenylate (cA4) in both pre- and post-cleavage states. These structures establish the molecular basis of the long-range allosteric activation of TtCsm6 ribonuclease by cA4. cA4 binding induces significant conformational changes, including closure of the CARF domain, dimerization of the HTH domain, and reorganization of the R-X4-6-H motif within the HEPN domain. The cleavage of cA4 by the CARF domain restores each domain to a conformation similar to its apo state. Furthermore, we have identified hyperactive TtCsm6 variants that exhibit sustained cA4-activated RNase activity, showing great promise for their applications in genome editing and diagnostics.