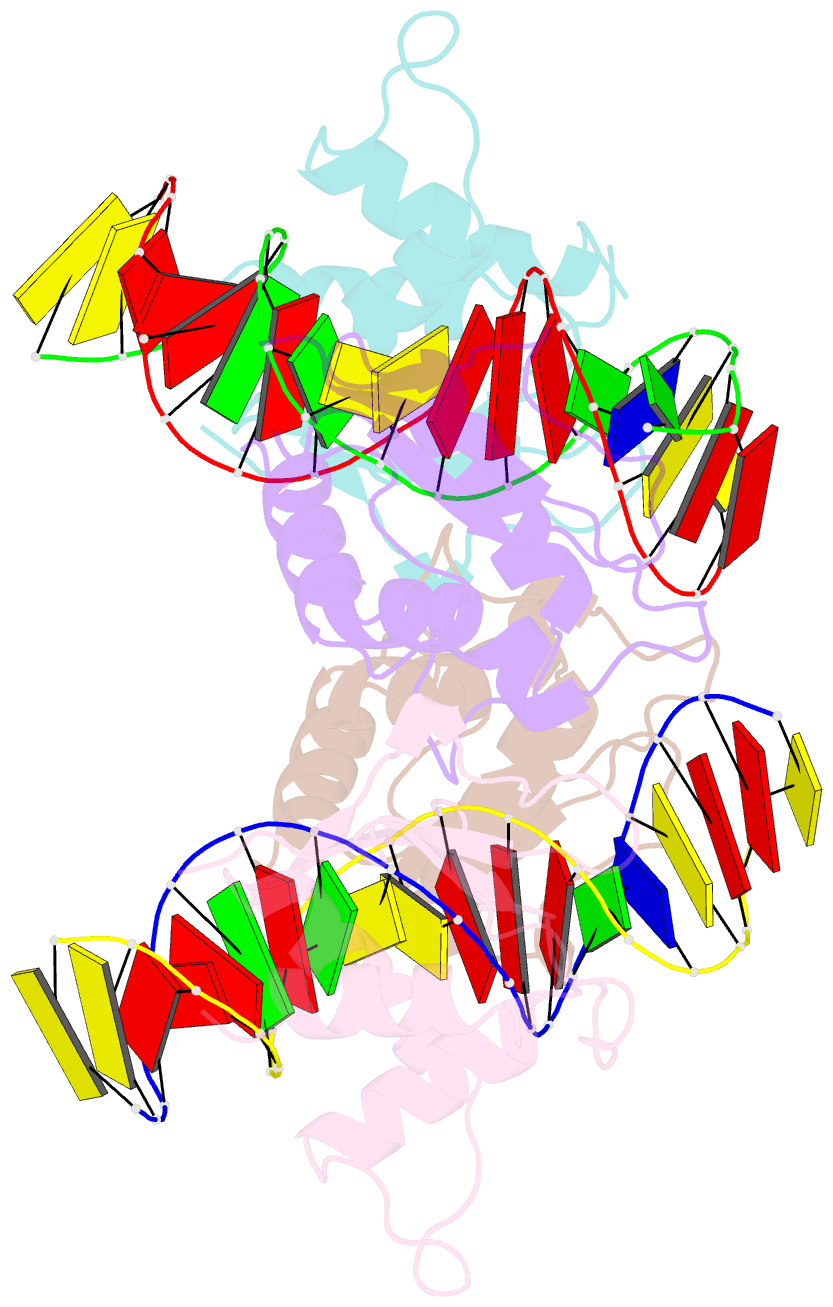
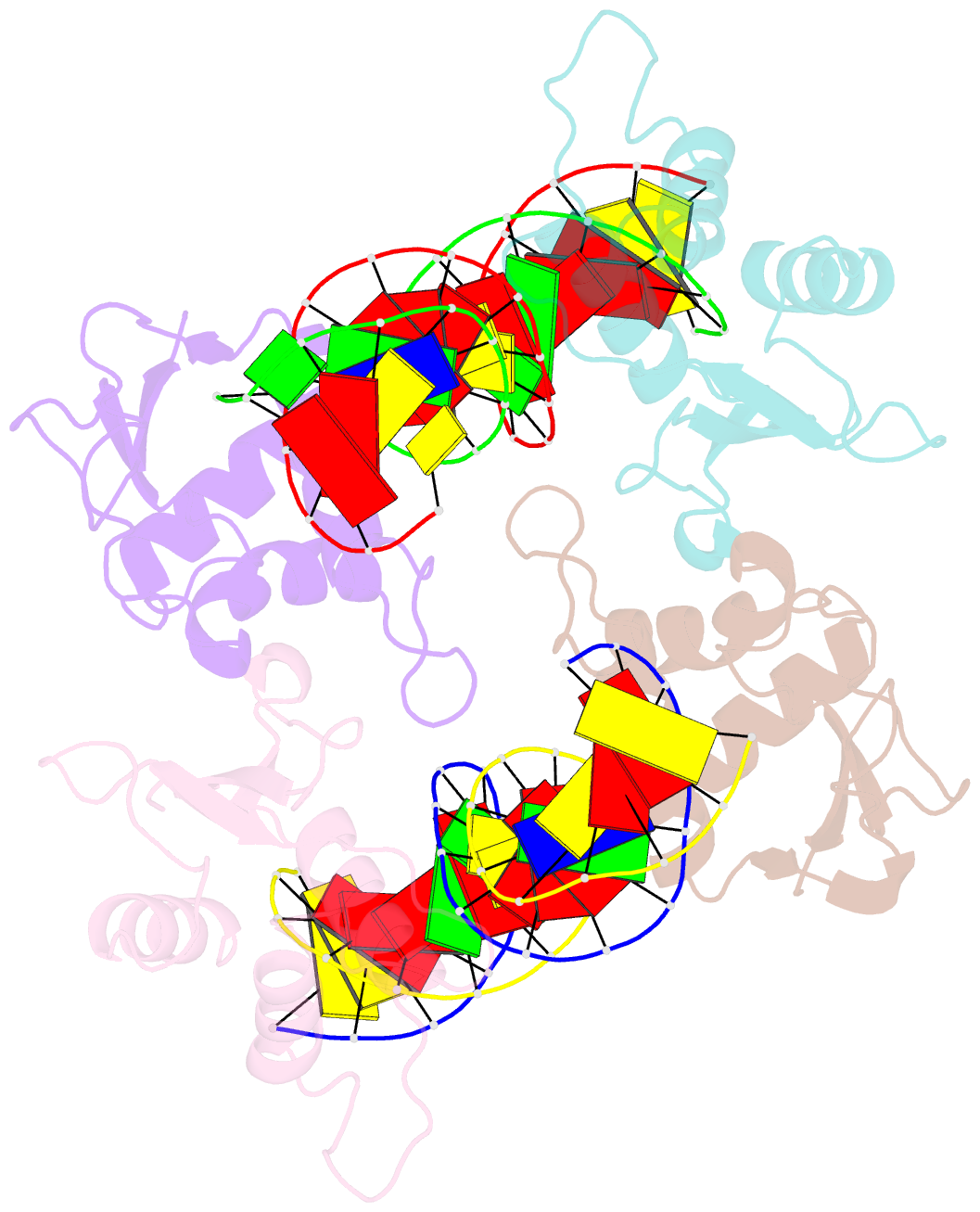
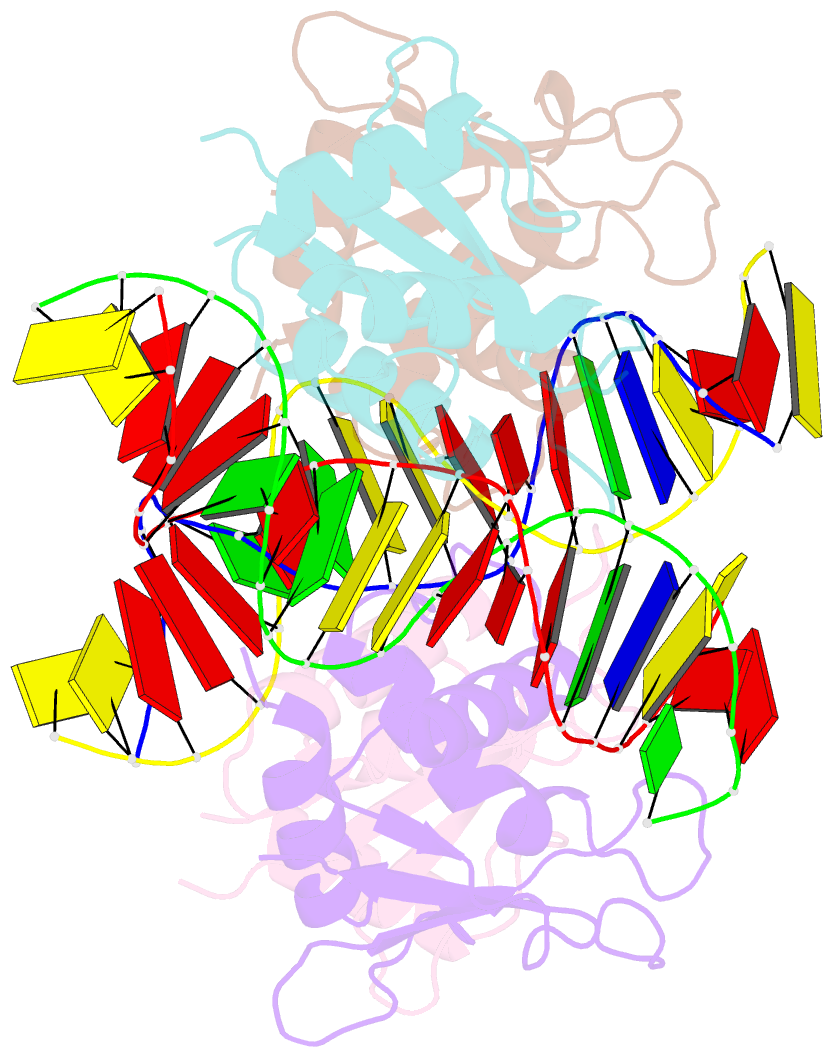
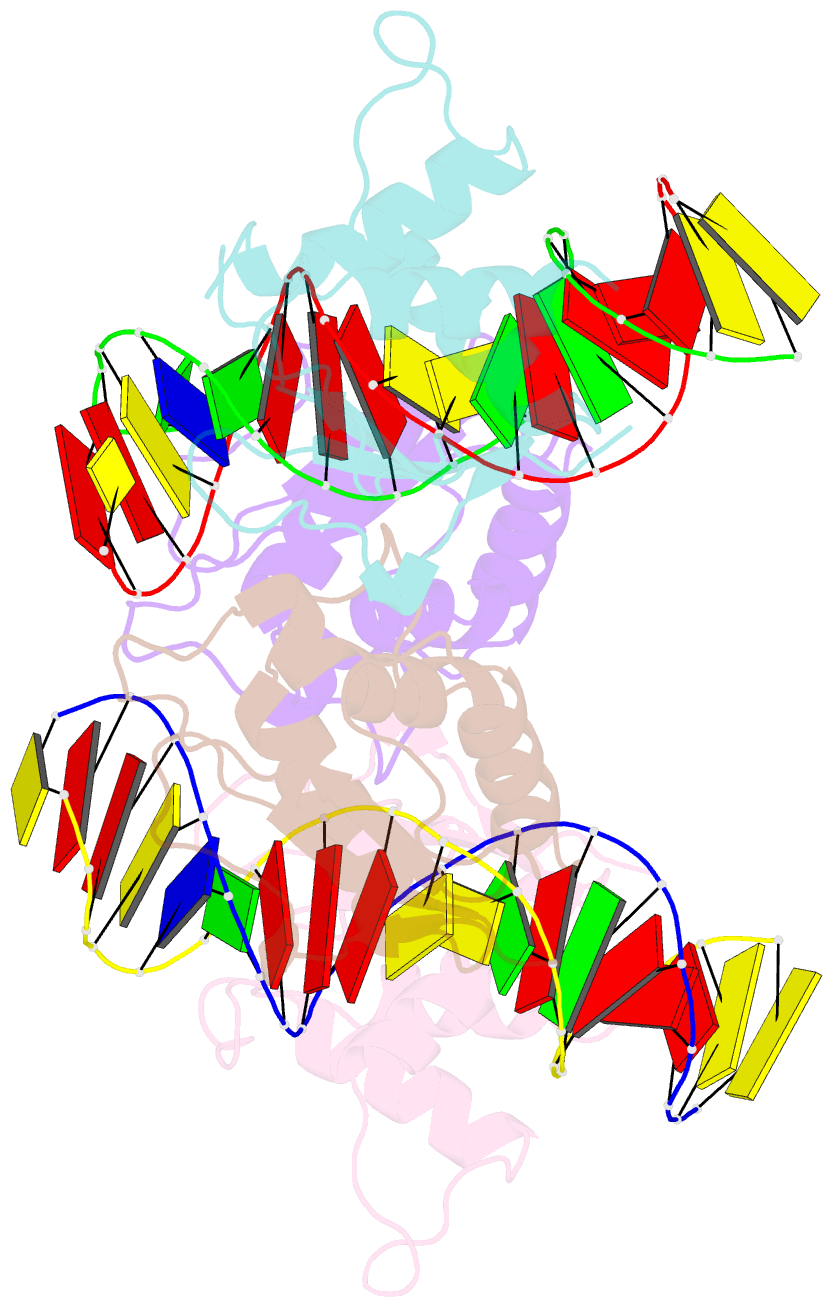
Summary information and primary citation
- PDB-id
- 8jkn; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription
- Method
- X-ray (2.92 Å)
- Summary
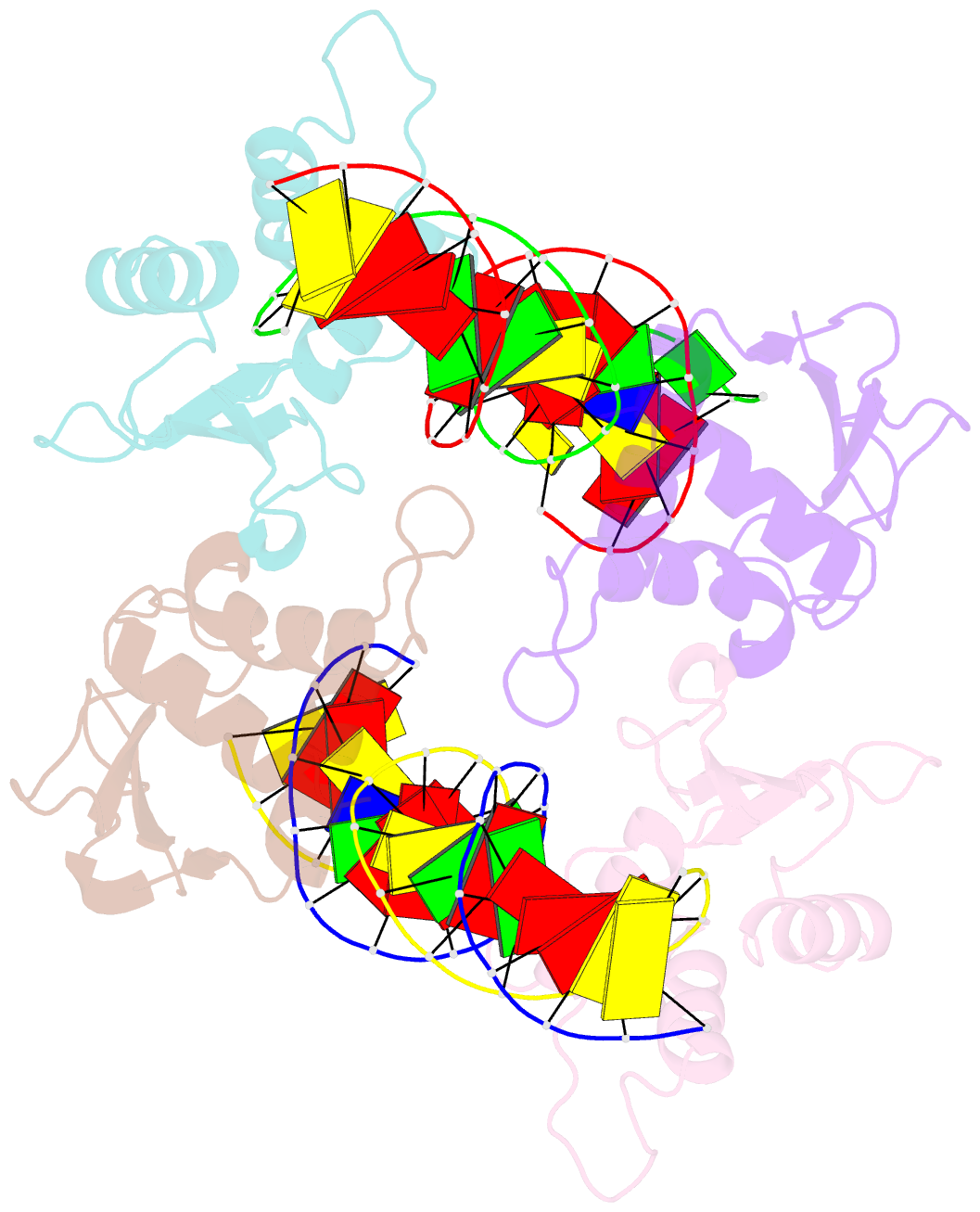
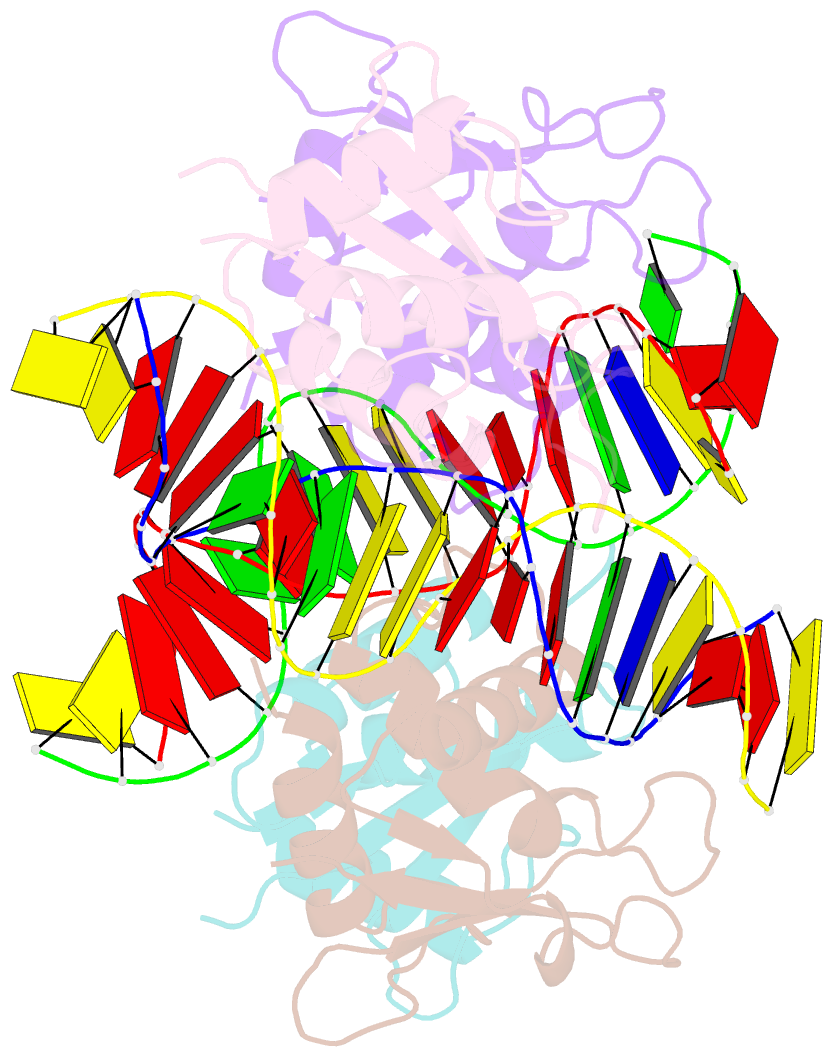
- T95r mutant irf4 DNA-binding domain bound to an DNA containing gaaa motif
- Reference
- Wang G, Feng X, Ding J (2023): "Molecular basis for the functional roles of the multimorphic T95R mutation of IRF4 causing human autosomal dominant combined immunodeficiency." Structure, 31, 1441. doi: 10.1016/j.str.2023.08.013.
- Abstract
- Interferon regulatory factor 4 (IRF4) is a transcription factor that regulates the development and function of immune cells. Recently, a new multimorphic mutation T95R was identified in the IRF4 DNA-binding domain (DBD) in patients with autosomal dominant combined immune deficiency. Here, we characterized the interactions of the wild-type IRF4-DBD (IRF4-DBDWT) and T95R mutant (IRF4-DBDT95R) with a canonical DNA sequence and several noncanonical DNA sequences. We found that compared to IRF4-DBDWT, IRF4-DBDT95R exhibits higher binding affinities for both canonical and noncanonical DNAs, with the highest preference for the noncanonical GATA sequence. The crystal structures of IRF4-DBDWT in complex with the GATA sequence and IRF4-DBDT95R in complexes with both canonical and noncanonical DNAs were determined, showing that the T95R mutation enhances the interactions of IRF4-DBDT95R with the canonical and noncanonical DNAs to achieve higher affinity and specificity. Collectively, our data provide the molecular basis for the gain-of-function and new function of IRF4T95R.