Summary information and primary citation
- PDB-id
- 8jla; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- gene regulation-DNA
- Method
- cryo-EM (3.44 Å)
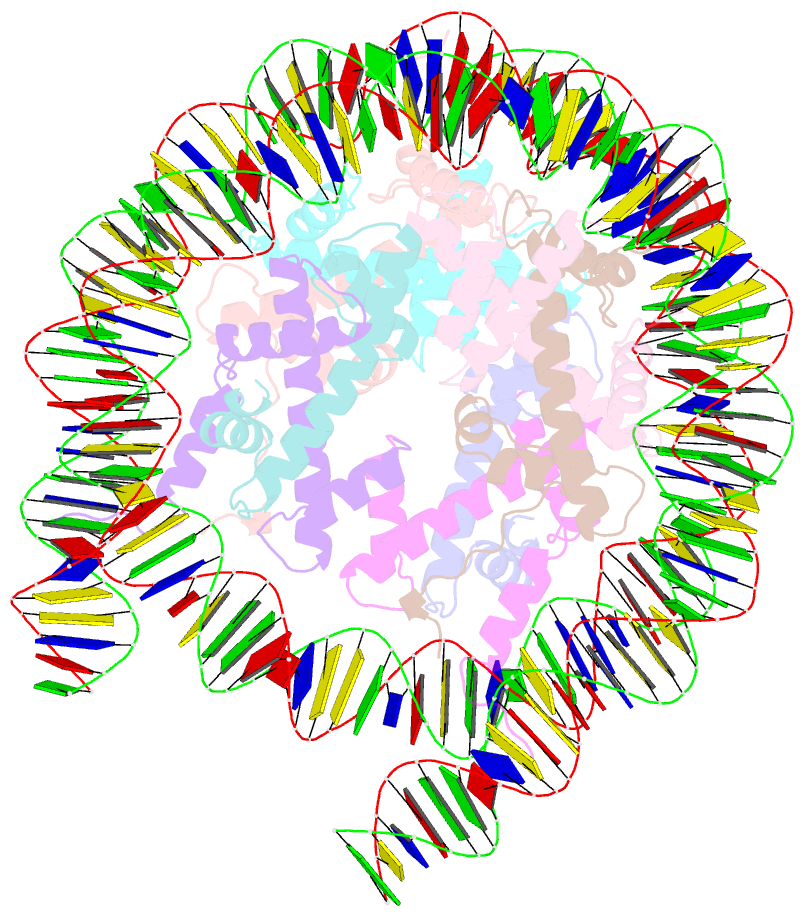
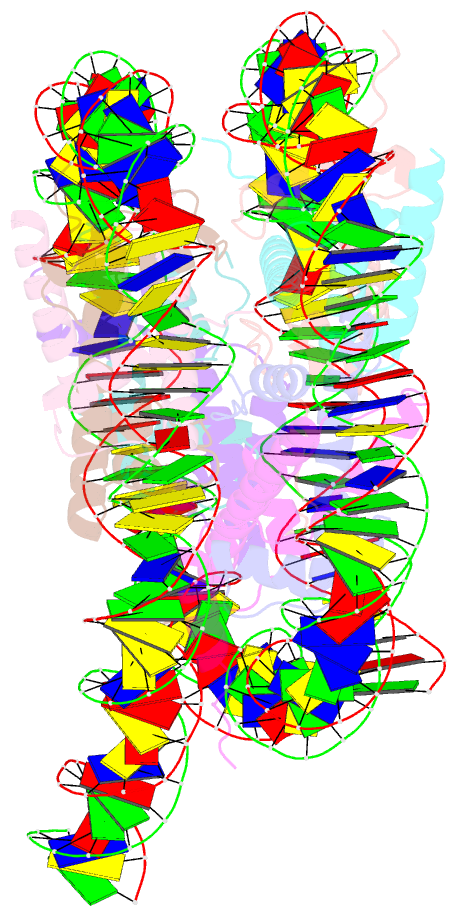
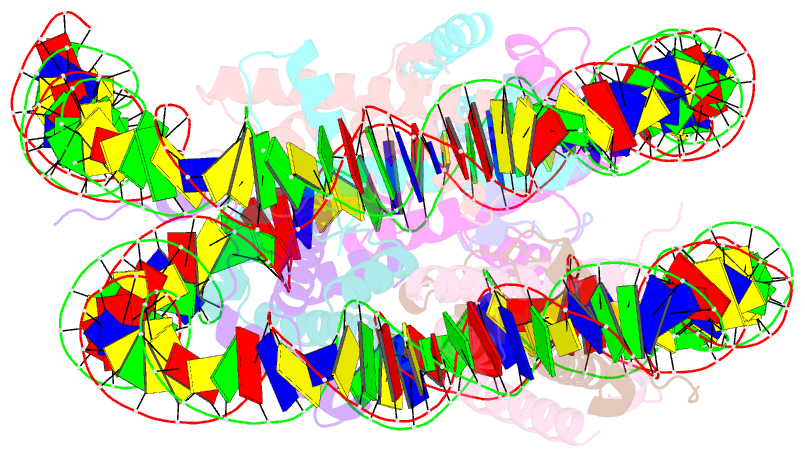
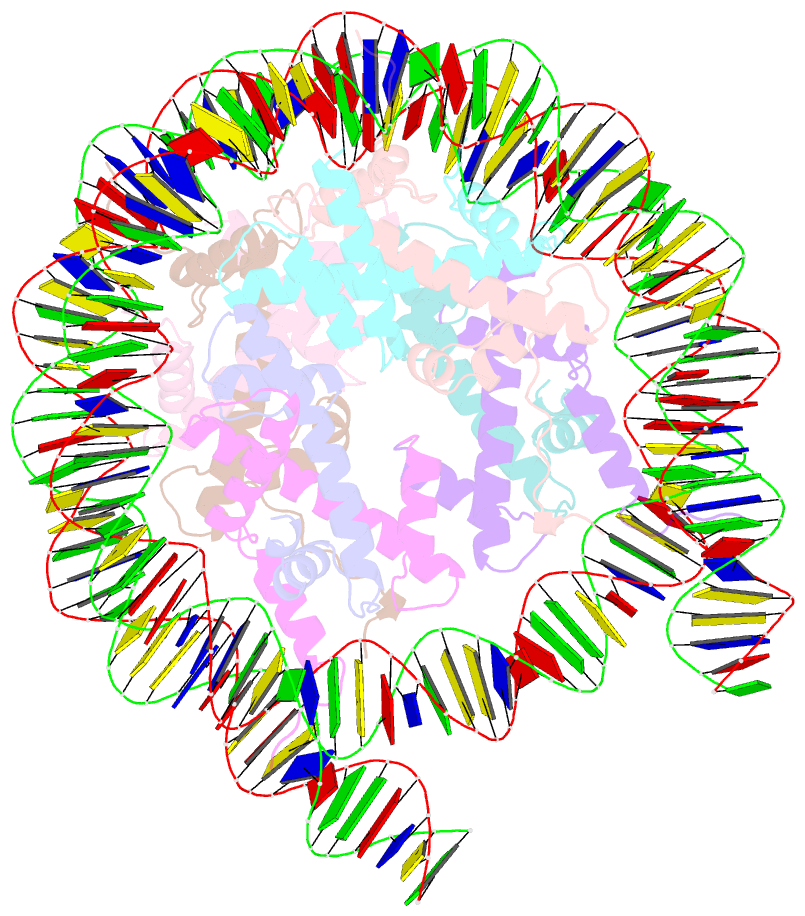
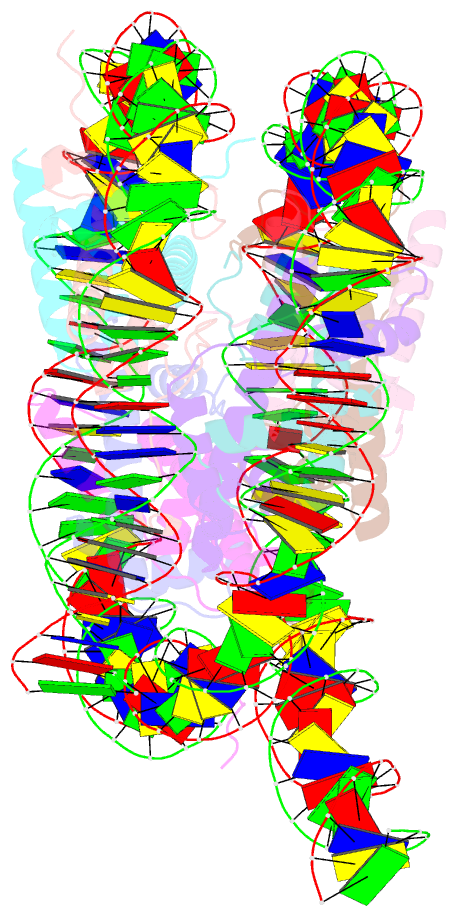
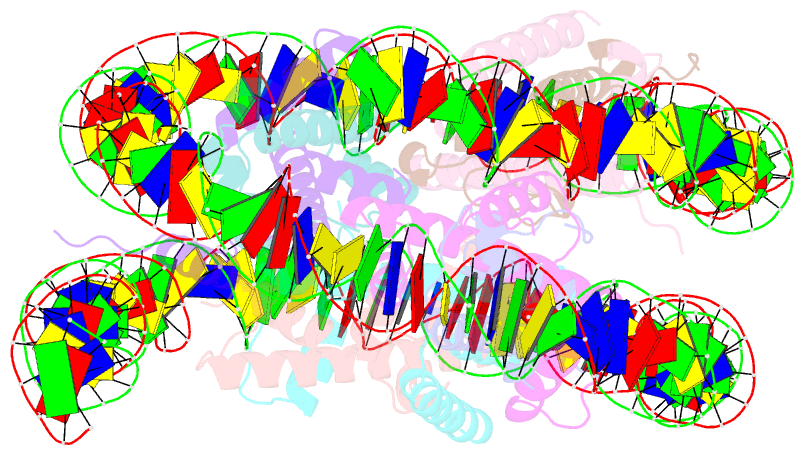
- Summary
- cryo-EM structure of the human nucleosome lacking n-terminal region of h2a, h2b, h3, and h4
- Reference
- Oishi T, Hatazawa S, Kujirai T, Kato J, Kobayashi Y, Ogasawara M, Akatsu M, Ehara H, Sekine SI, Hayashi G, Takizawa Y, Kurumizaka H (2023): "Contributions of histone tail clipping and acetylation in nucleosome transcription by RNA polymerase II." Nucleic Acids Res., 51, 10364-10374. doi: 10.1093/nar/gkad754.
- Abstract
- The N-terminal tails of histones protrude from the nucleosome core and are target sites for histone modifications, such as acetylation and methylation. Histone acetylation is considered to enhance transcription in chromatin. However, the contribution of the histone N-terminal tail to the nucleosome transcription by RNA polymerase II (RNAPII) has not been clarified. In the present study, we reconstituted nucleosomes lacking the N-terminal tail of each histone, H2A, H2B, H3 or H4, and performed RNAPII transcription assays. We found that the N-terminal tail of H3, but not H2A, H2B and H4, functions in RNAPII pausing at the SHL(-5) position of the nucleosome. Consistently, the RNAPII transcription assay also revealed that the nucleosome containing N-terminally acetylated H3 drastically alleviates RNAPII pausing at the SHL(-5) position. In addition, the H3 acetylated nucleosome produced increased amounts of the run-off transcript. These results provide important evidence that the H3 N-terminal tail plays a role in RNAPII pausing at the SHL(-5) position of the nucleosome, and its acetylation directly alleviates this nucleosome barrier.