Summary information and primary citation
- PDB-id
- 8jne; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- cryo-EM (4.68 Å)
- Summary
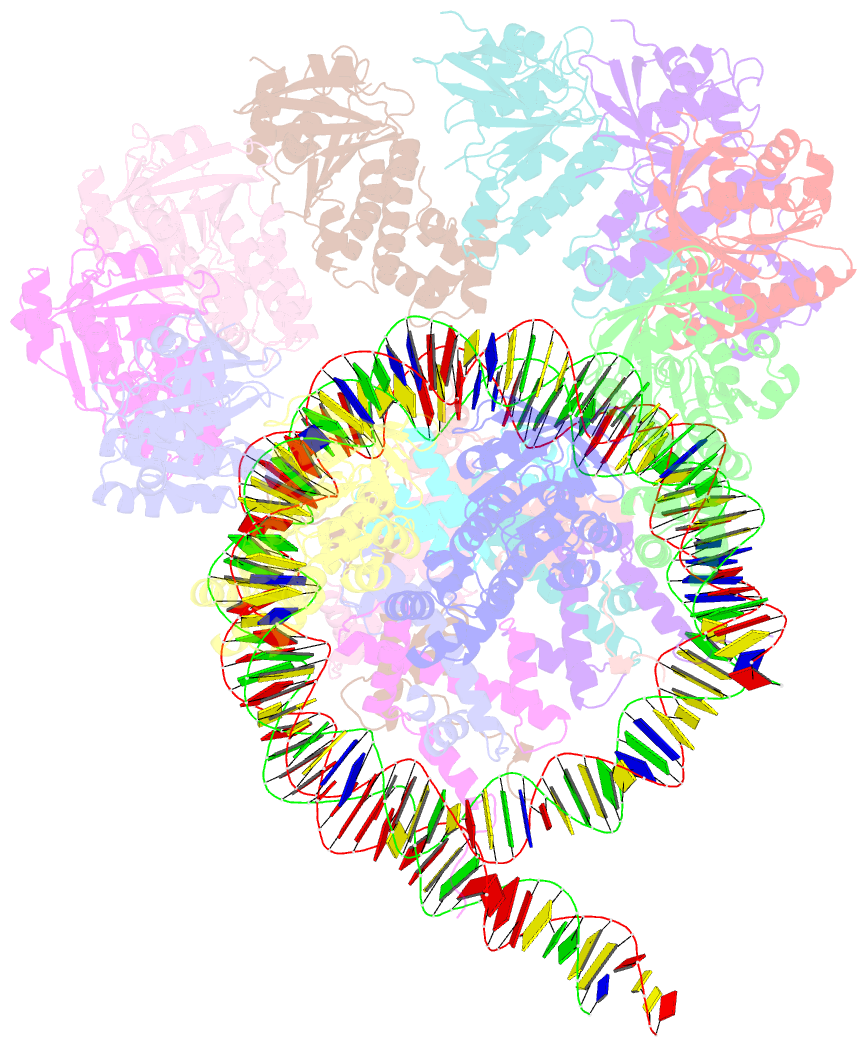
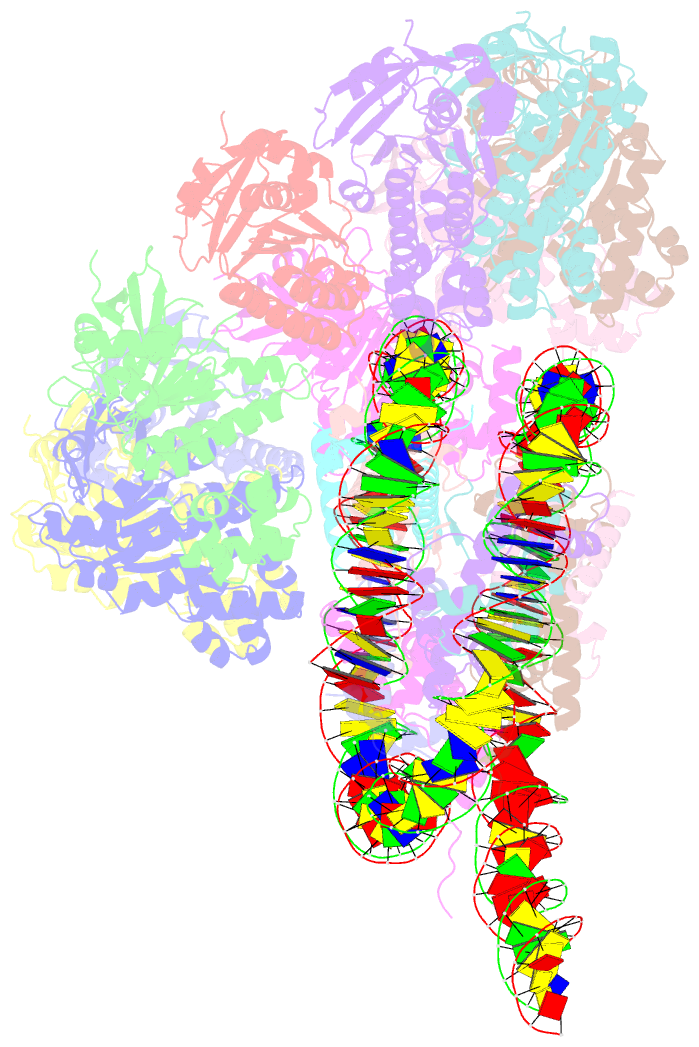
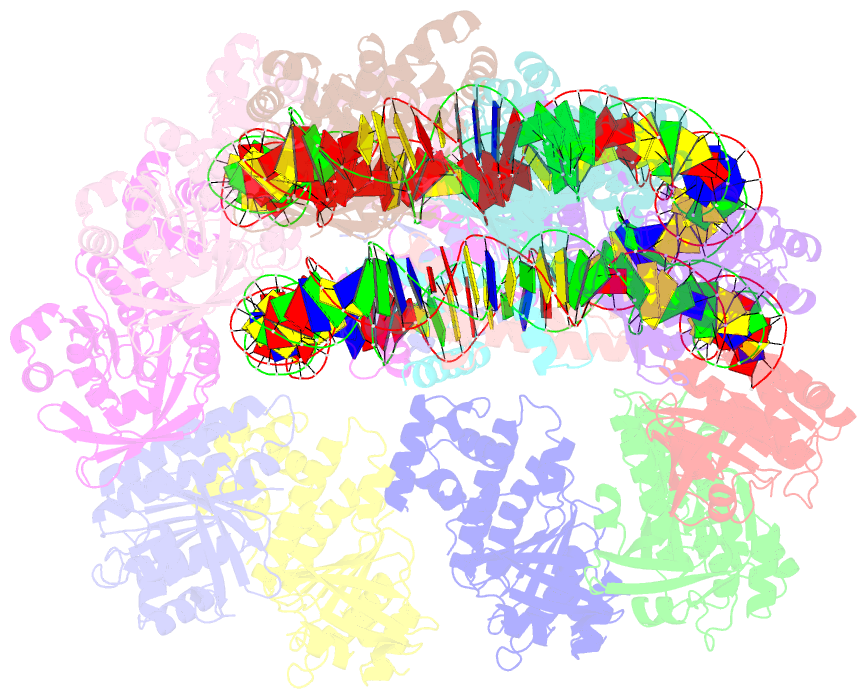
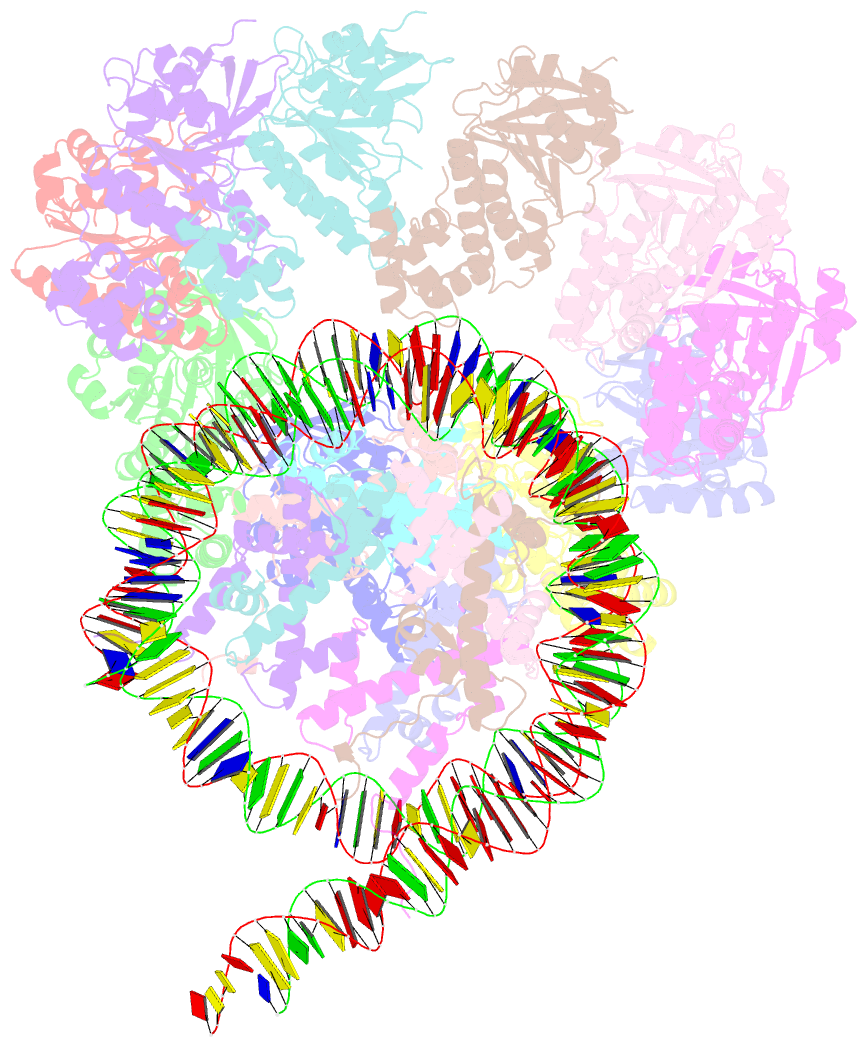
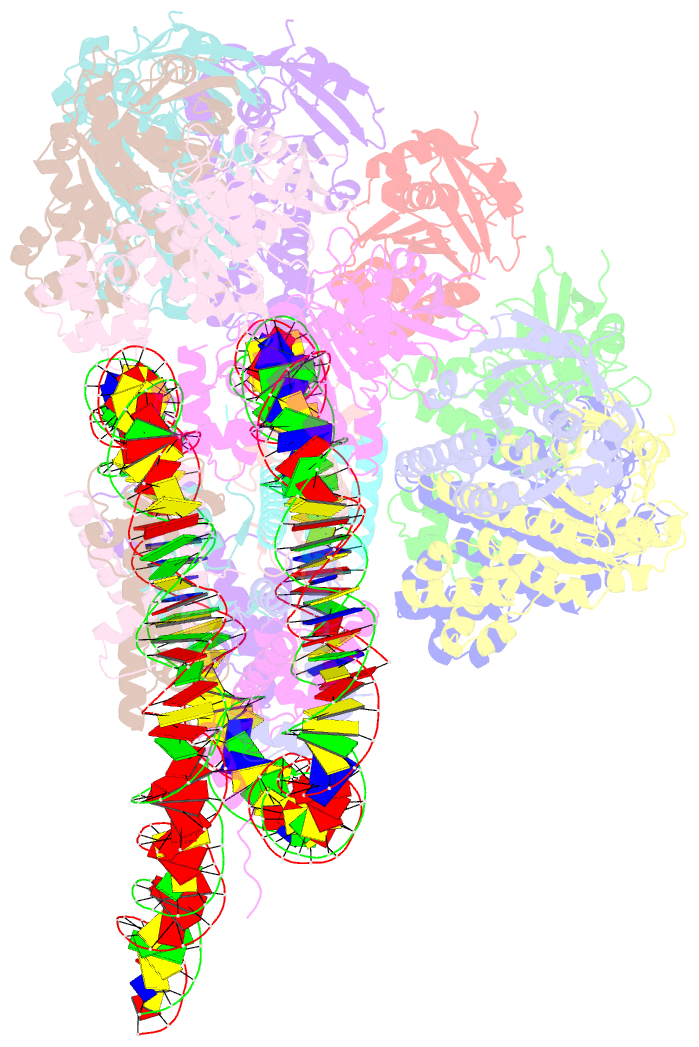
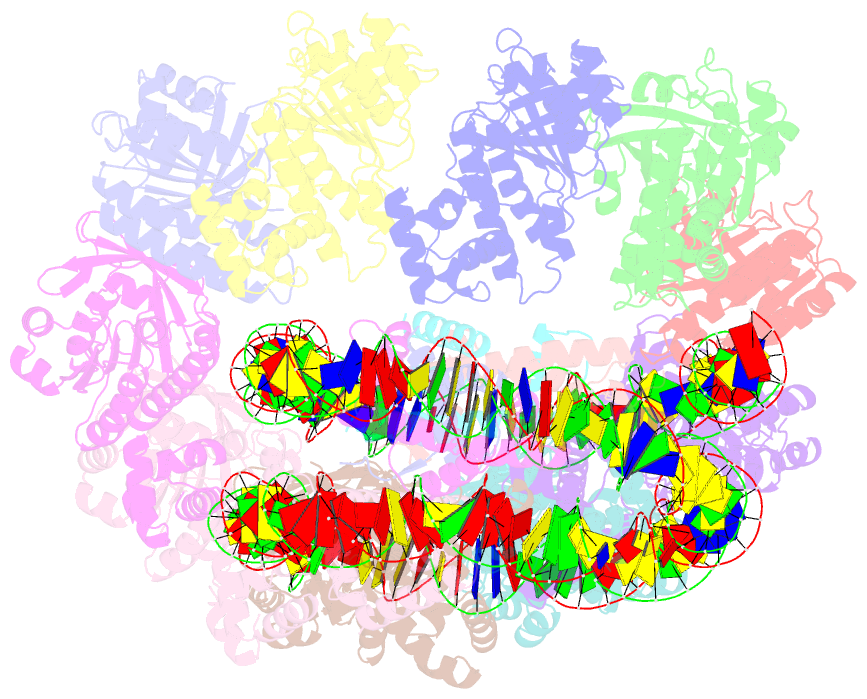
- The cryo-EM structure of the decameric rad51 ring bound to the nucleosome without the linker DNA binding
- Reference
- Shioi T, Hatazawa S, Oya E, Hosoya N, Kobayashi W, Ogasawara M, Kobayashi T, Takizawa Y, Kurumizaka H (2024): "Cryo-EM structures of RAD51 assembled on nucleosomes containing a DSB site." Nature, 628, 212-220. doi: 10.1038/s41586-024-07196-4.
- Abstract
- RAD51 is the central eukaryotic recombinase required for meiotic recombination and mitotic repair of double-strand DNA breaks (DSBs)1,2. However, the mechanism by which RAD51 functions at DSB sites in chromatin has remained elusive. Here we report the cryo-electron microscopy structures of human RAD51-nucleosome complexes, in which RAD51 forms ring and filament conformations. In the ring forms, the N-terminal lobe domains (NLDs) of RAD51 protomers are aligned on the outside of the RAD51 ring, and directly bind to the nucleosomal DNA. The nucleosomal linker DNA that contains the DSB site is recognized by the L1 and L2 loops-active centres that face the central hole of the RAD51 ring. In the filament form, the nucleosomal DNA is peeled by the RAD51 filament extension, and the NLDs of RAD51 protomers proximal to the nucleosome bind to the remaining nucleosomal DNA and histones. Mutations that affect nucleosome-binding residues of the RAD51 NLD decrease nucleosome binding, but barely affect DNA binding in vitro. Consistently, yeast Rad51 mutants with the corresponding mutations are substantially defective in DNA repair in vivo. These results reveal an unexpected function of the RAD51 NLD, and explain the mechanism by which RAD51 associates with nucleosomes, recognizes DSBs and forms the active filament in chromatin.