Summary information and primary citation
- PDB-id
- 8omr; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein
- Method
- cryo-EM (3.3 Å)
- Summary
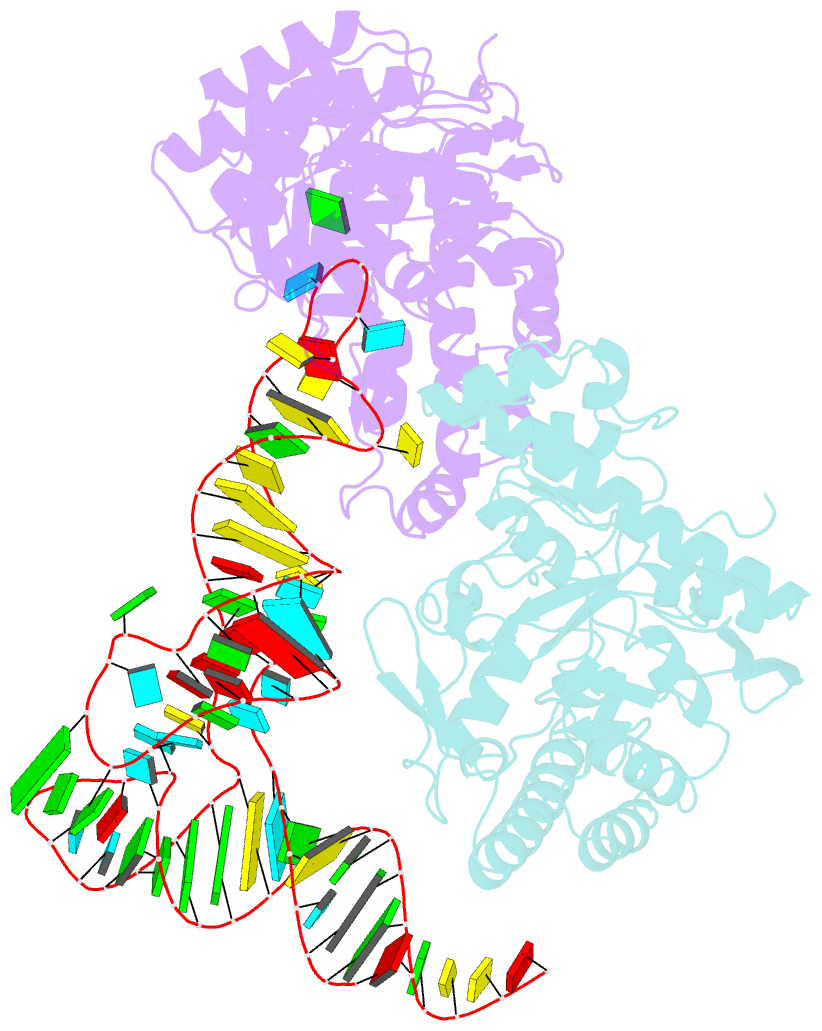
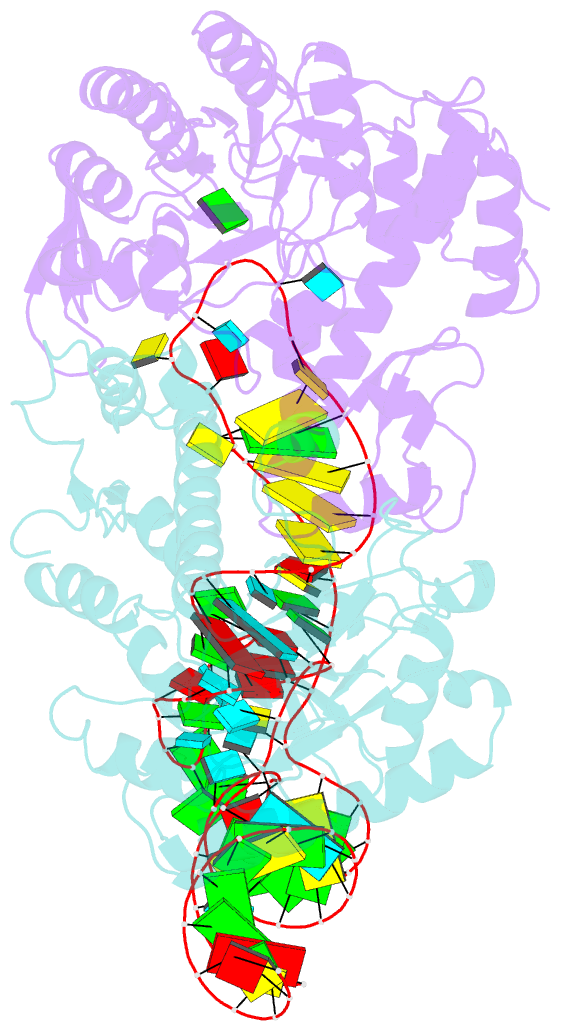
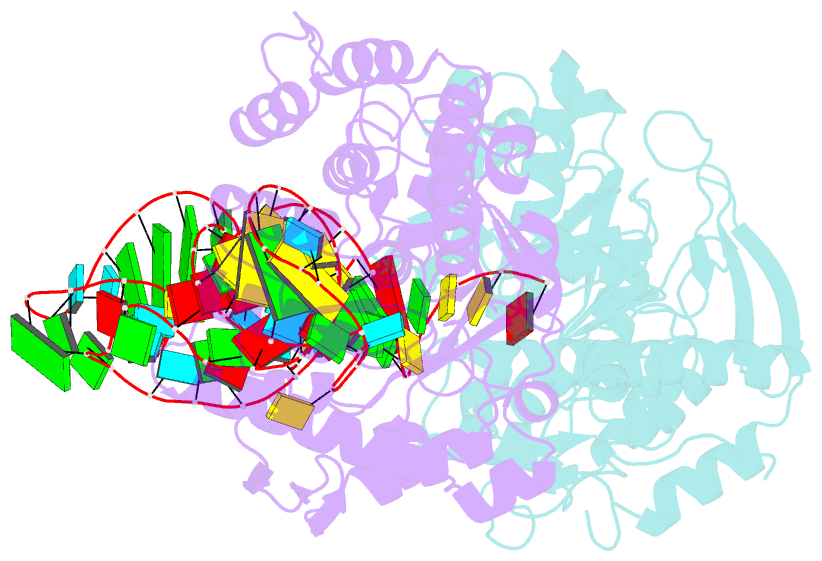
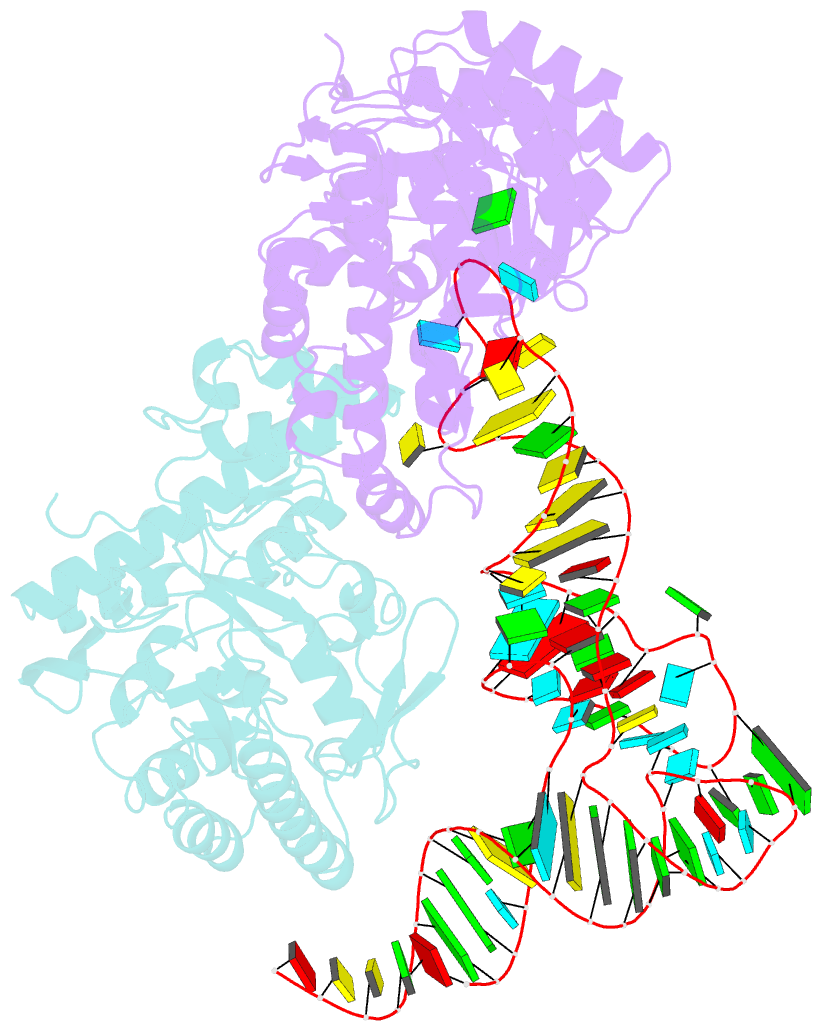
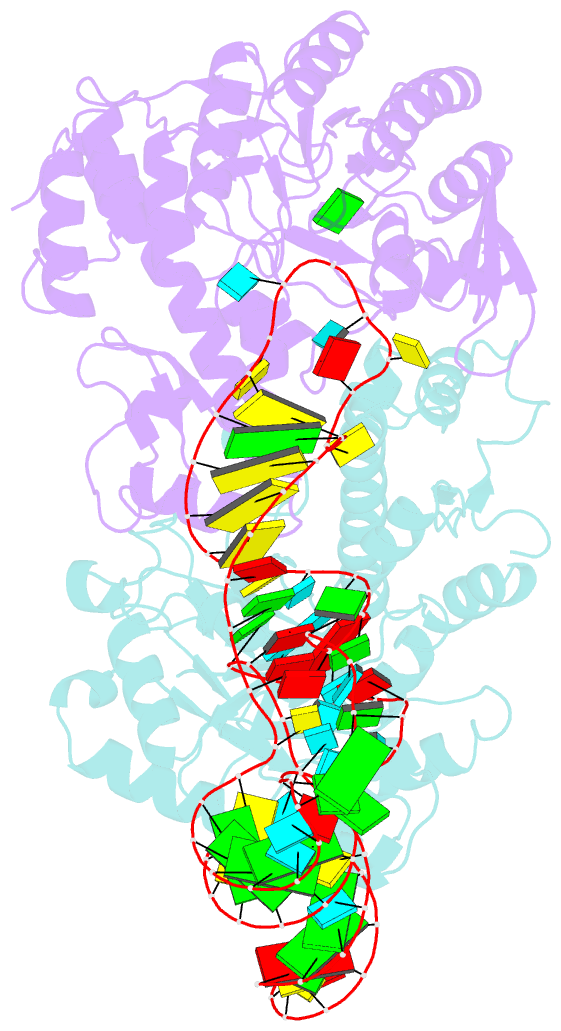
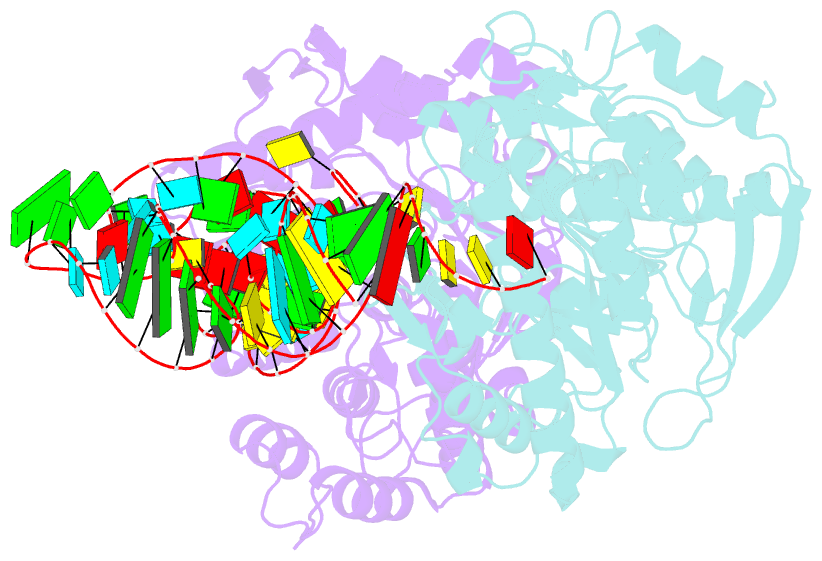
- Human trna guanine transglycosylase (tgt) bound to trnaasp
- Reference
- Sievers K, Neumann P, Susac L, Da Vela S, Graewert M, Trowitzsch S, Svergun D, Tampe R, Ficner R (2024): "Structural and functional insights into tRNA recognition by human tRNA guanine transglycosylase." Structure, 32, 316. doi: 10.1016/j.str.2023.12.006.
- Abstract
- Eukaryotic tRNA guanine transglycosylase (TGT) is an RNA-modifying enzyme which catalyzes the base exchange of the genetically encoded guanine 34 of tRNAsAsp,Asn,His,Tyr for queuine, a hypermodified 7-deazaguanine derivative. Eukaryotic TGT is a heterodimer comprised of a catalytic and a non-catalytic subunit. While binding of the tRNA anticodon loop to the active site is structurally well understood, the contribution of the non-catalytic subunit to tRNA binding remained enigmatic, as no complex structure with a complete tRNA was available. Here, we report a cryo-EM structure of eukaryotic TGT in complex with a complete tRNA, revealing the crucial role of the non-catalytic subunit in tRNA binding. We decipher the functional significance of these additional tRNA-binding sites, analyze solution state conformation, flexibility, and disorder of apo TGT, and examine conformational transitions upon tRNA binding.