Summary information and primary citation
- PDB-id
- 8op2; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- viral protein
- Method
- cryo-EM (2.8 Å)
- Summary
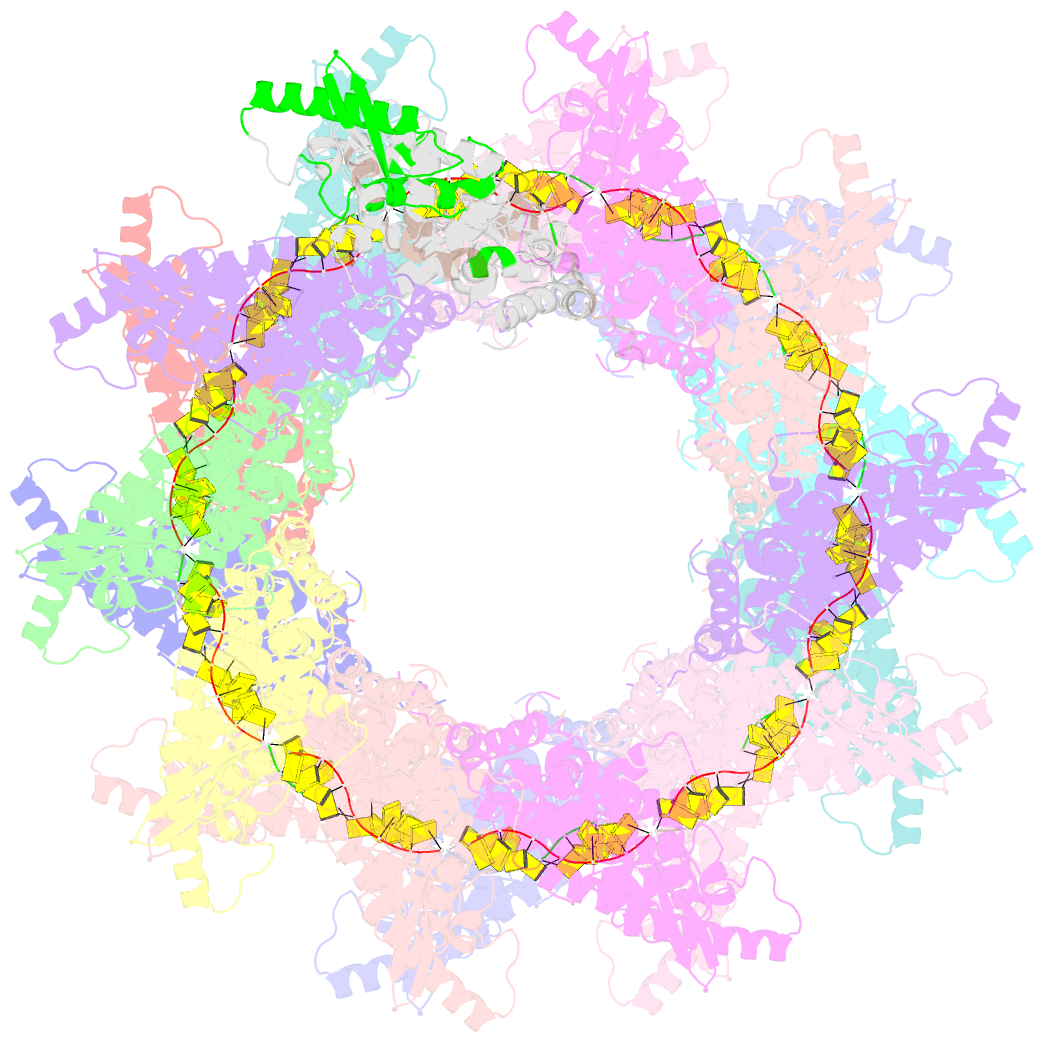
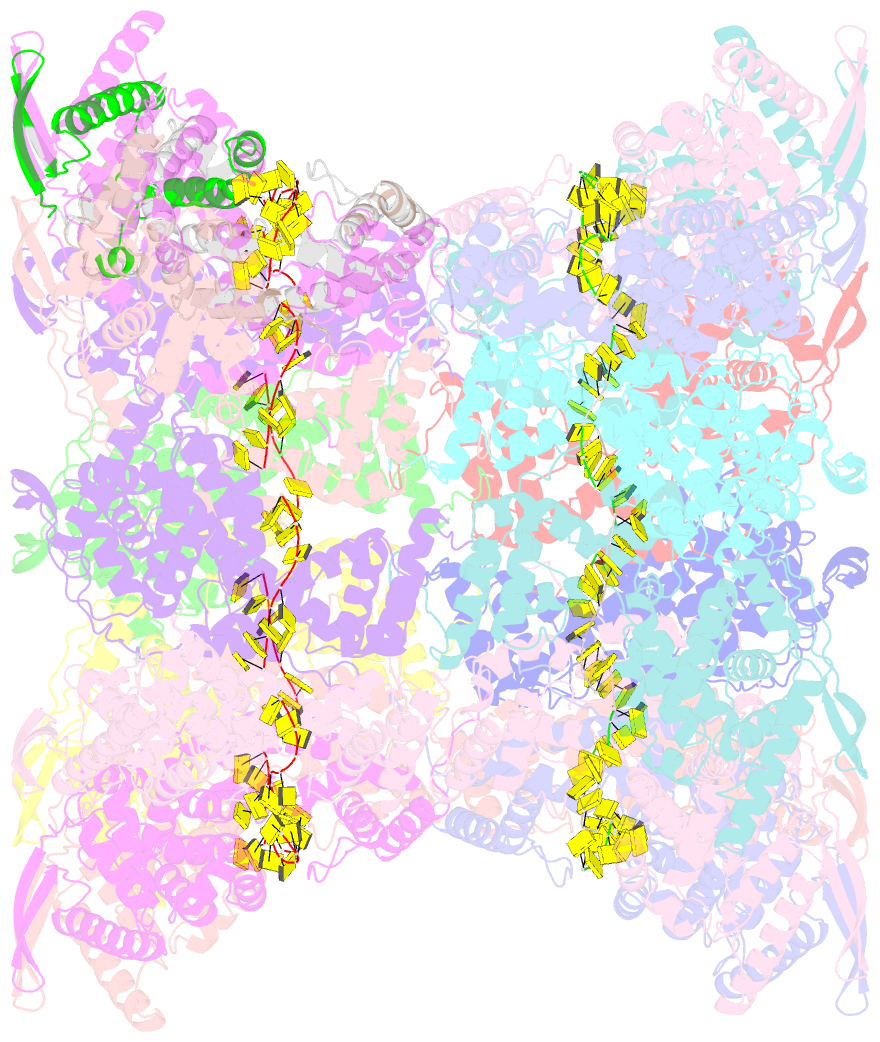
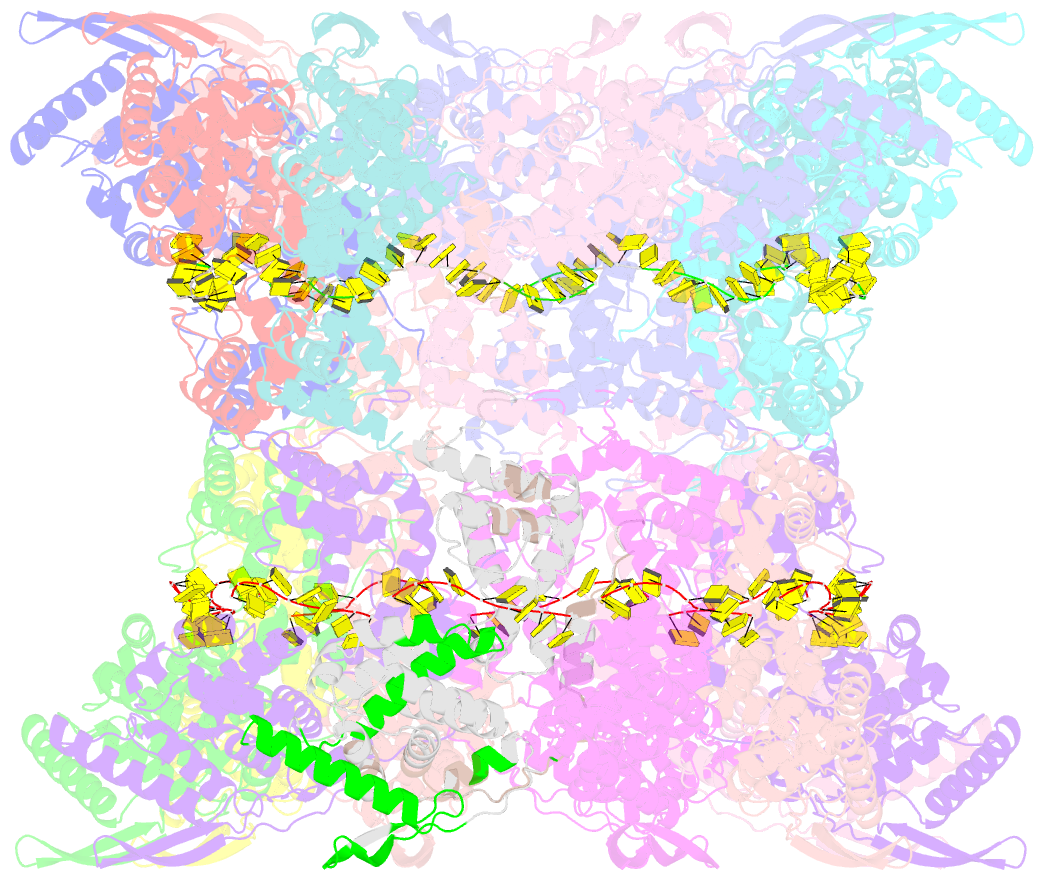
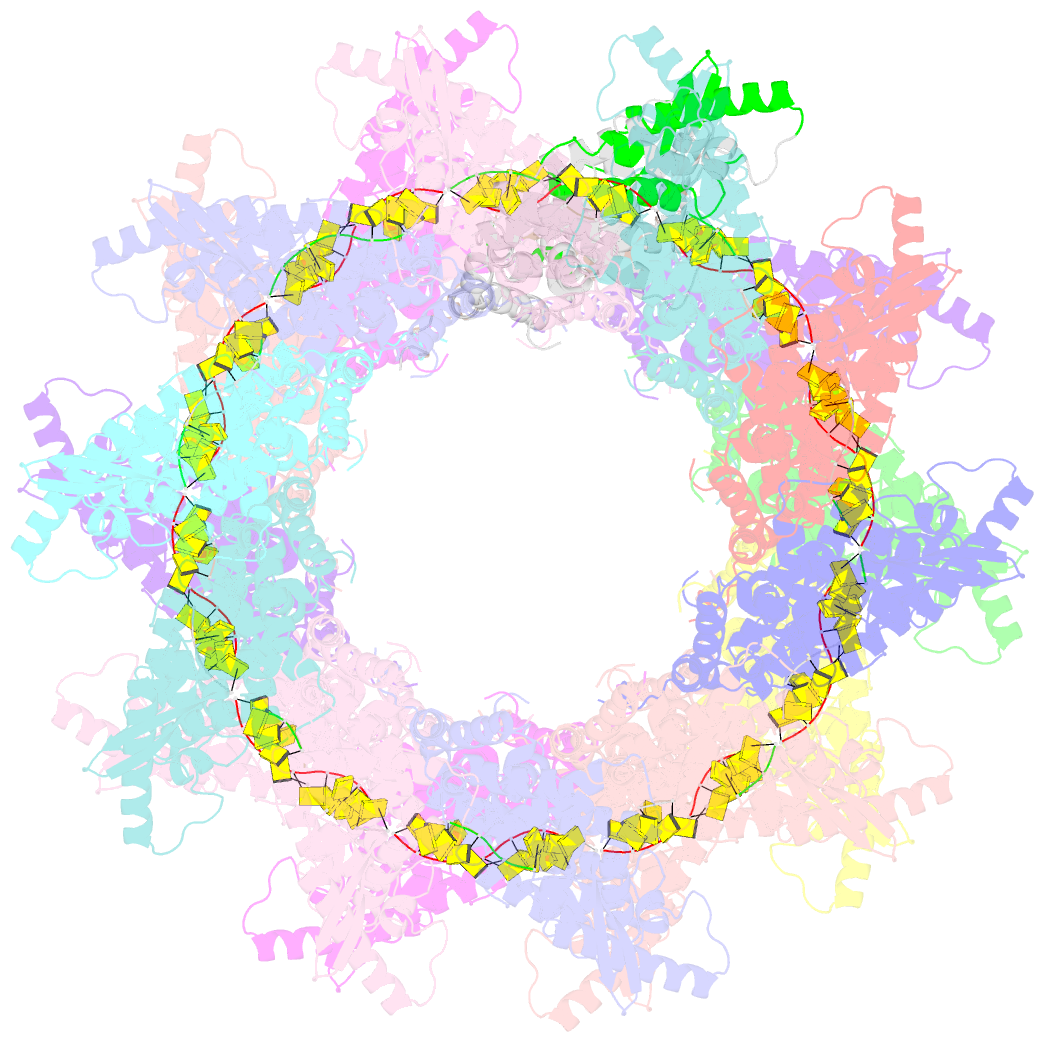
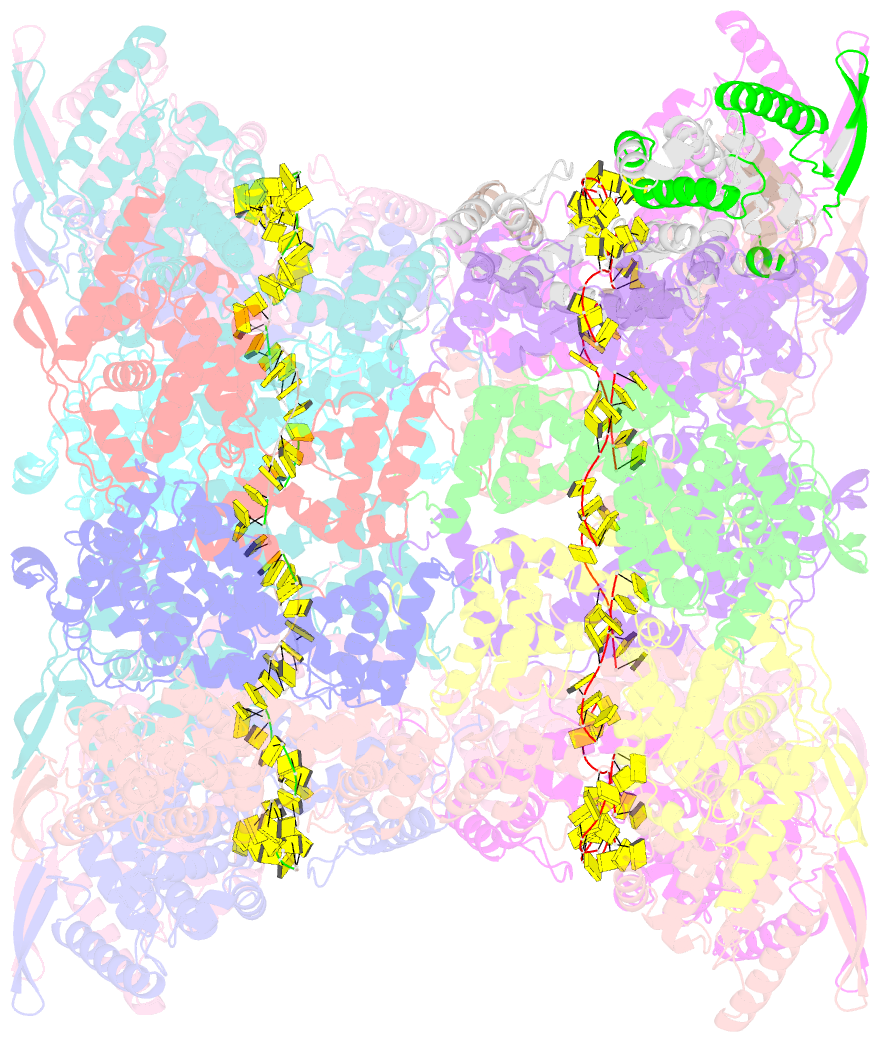
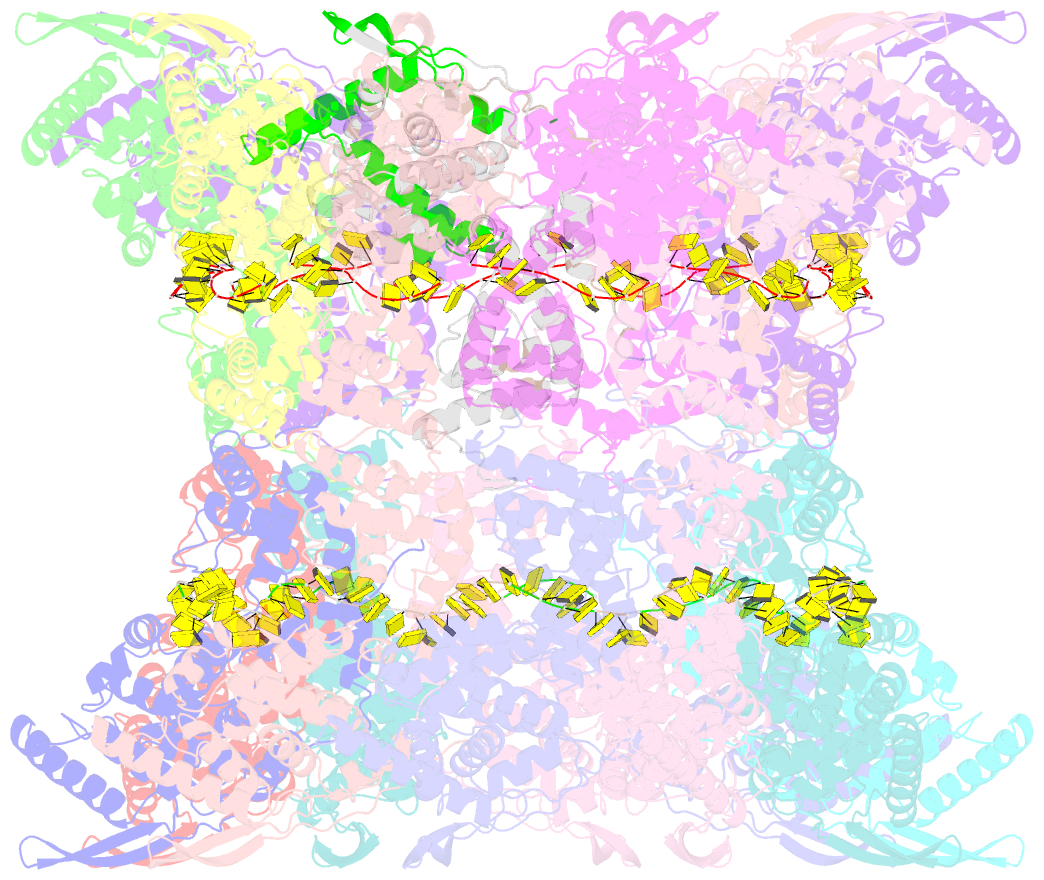
- Stacks of nucleocapsid rings of the n1-370 mutant of the human respiratory syncytial virus
- Reference
- Gonnin L, Desfosses A, Bacia-Verloop M, Chevret D, Galloux M, Eleouet JF, Gutsche I (2023): "Structural landscape of the respiratory syncytial virus nucleocapsids." Nat Commun, 14, 5732. doi: 10.1038/s41467-023-41439-8.
- Abstract
- Human Respiratory Syncytial Virus (HRSV) is a prevalent cause of severe respiratory infections in children and the elderly. The helical HRSV nucleocapsid is a template for the viral RNA synthesis and a scaffold for the virion assembly. This cryo-electron microscopy analysis reveals the non-canonical arrangement of the HRSV nucleocapsid helix, composed of 16 nucleoproteins per asymmetric unit, and the resulting systematic variations in the RNA accessibility. We demonstrate that this unique helical symmetry originates from longitudinal interactions by the C-terminal arm of the HRSV nucleoprotein. We explore the polymorphism of the nucleocapsid-like assemblies, report five structures of the full-length particles and two alternative arrangements formed by a C-terminally truncated nucleoprotein mutant, and demonstrate the functional importance of the identified longitudinal interfaces. We put all these findings in the context of the HRSV RNA synthesis machinery and delineate the structural basis for its further investigation.