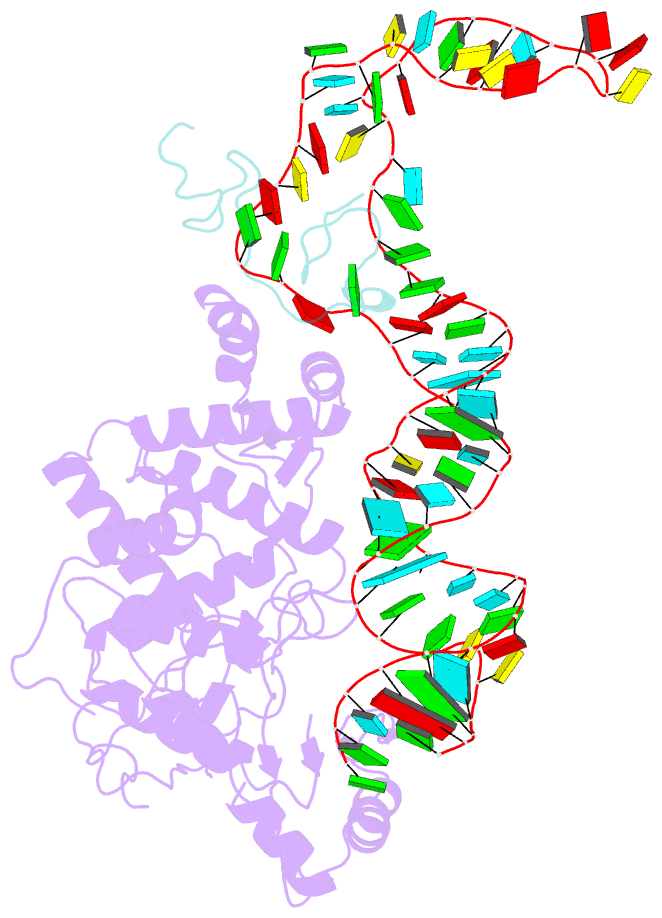
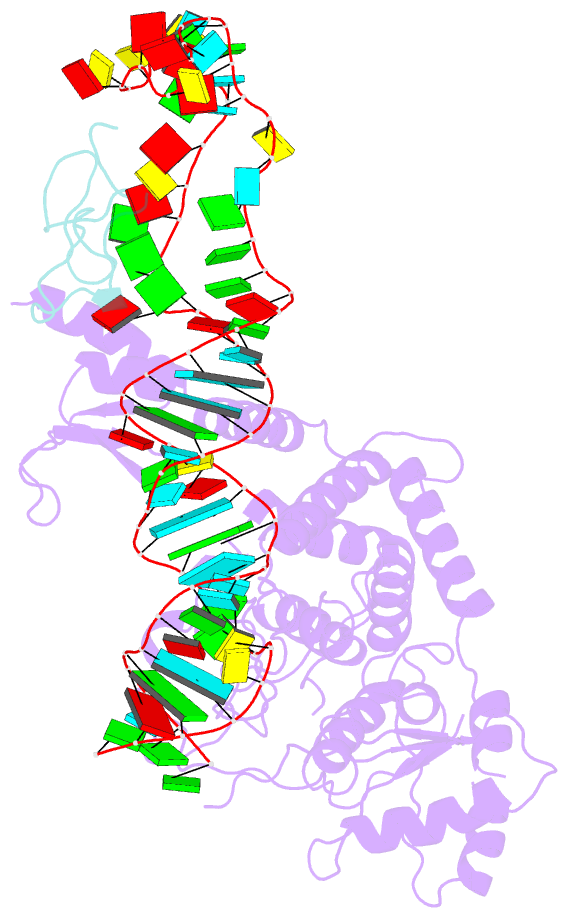
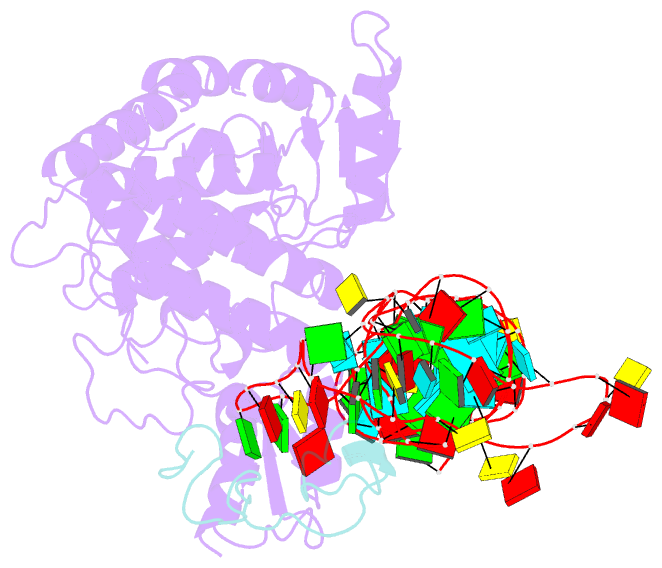
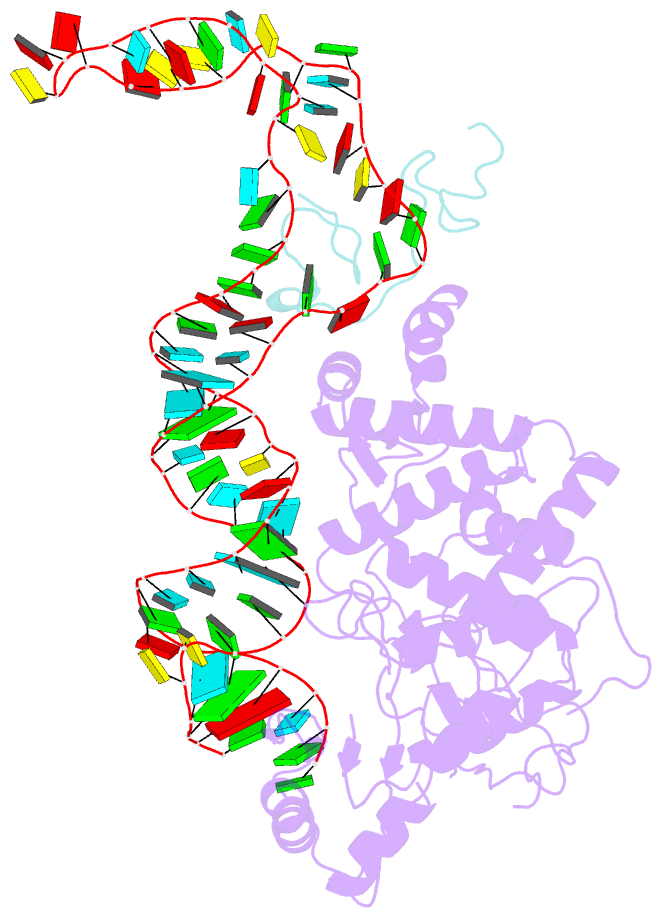
Summary information and primary citation
- PDB-id
- 8ost; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- cell cycle
- Method
- cryo-EM (3.69 Å)
- Summary
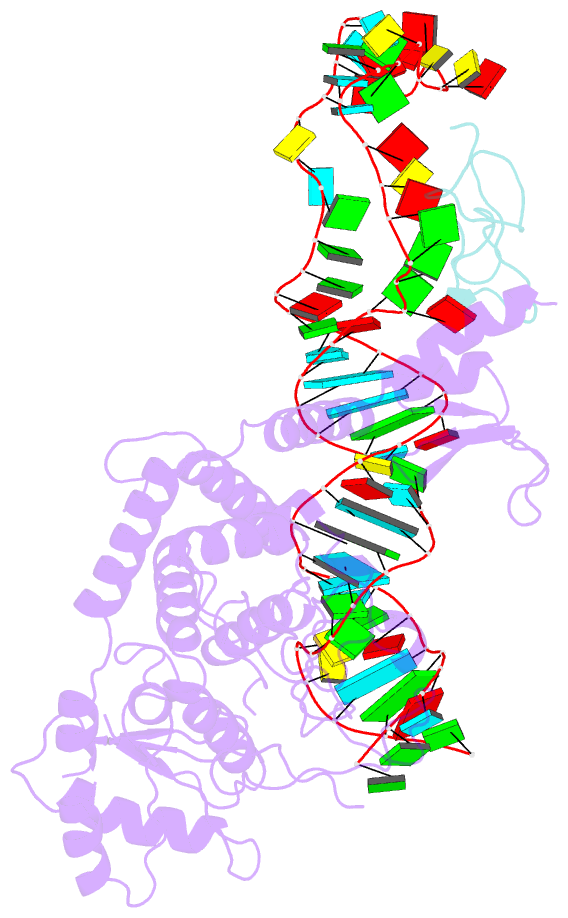
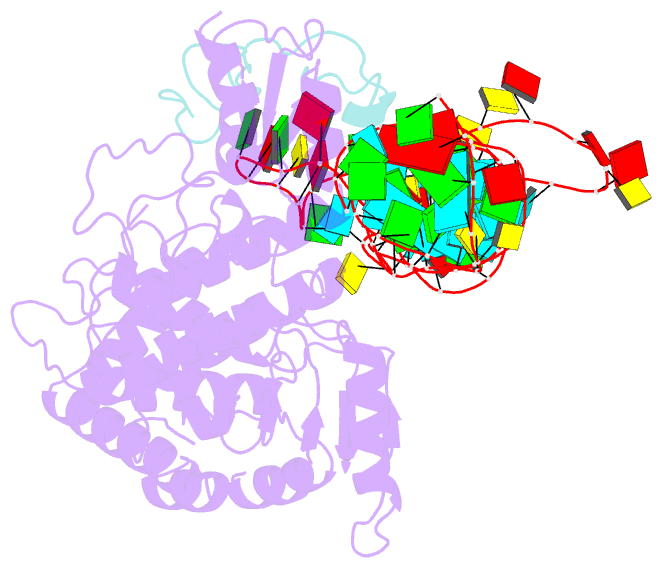
- Structure of human terminal uridylyltransferase 4 (tut4, zcchc11) in complex with pre-let7g mirna and lin28a
- Reference
- Yi G, Ye M, Carrique L, El-Sagheer A, Brown T, Norbury CJ, Zhang P, Gilbert RJC (2024): "Structural basis for activity switching in polymerases determining the fate of let-7 pre-miRNAs." Nat.Struct.Mol.Biol., 31, 1426-1438. doi: 10.1038/s41594-024-01357-9.
- Abstract
- Tumor-suppressor let-7 pre-microRNAs (miRNAs) are regulated by terminal uridylyltransferases TUT7 and TUT4 that either promote let-7 maturation by adding a single uridine nucleotide to the pre-miRNA 3' end or mark them for degradation by the addition of multiple uridines. Oligo-uridylation is increased in cells by enhanced TUT7/4 expression and especially by the RNA-binding pluripotency factor LIN28A. Using cryogenic electron microscopy, we captured high-resolution structures of active forms of TUT7 alone, of TUT7 plus pre-miRNA and of both TUT7 and TUT4 bound with pre-miRNA and LIN28A. Our structures reveal that pre-miRNAs engage the enzymes in fundamentally different ways depending on the presence of LIN28A, which clamps them onto the TUTs to enable processive 3' oligo-uridylation. This study reveals the molecular basis for mono- versus oligo-uridylation by TUT7/4, as determined by the presence of LIN28A, and thus their mechanism of action in the regulation of cell fate and in cancer.