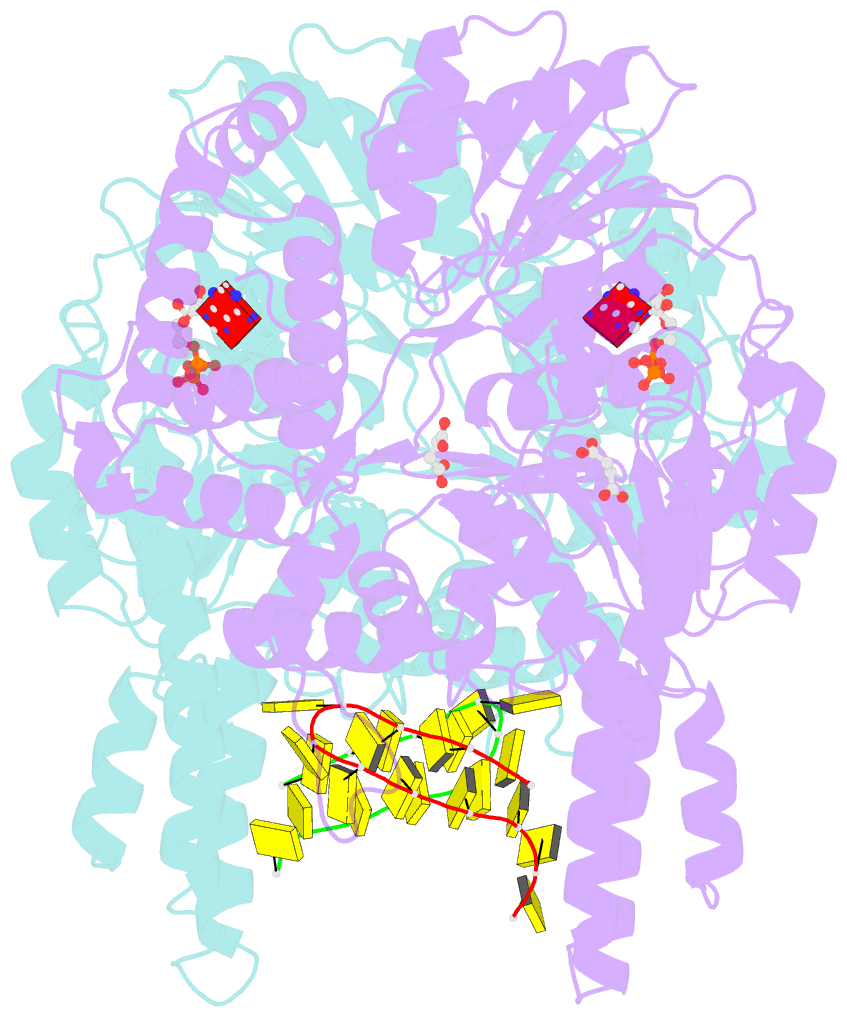
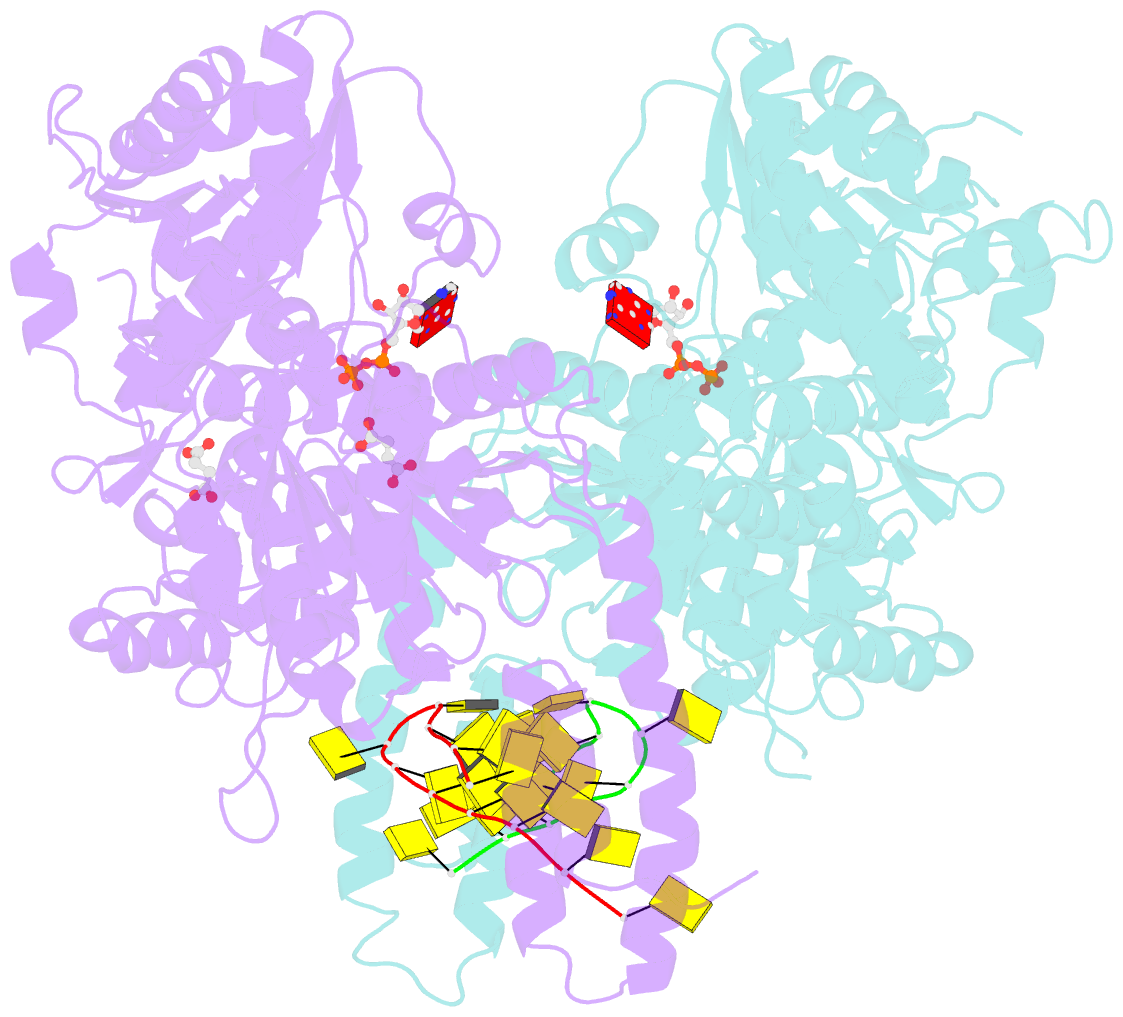
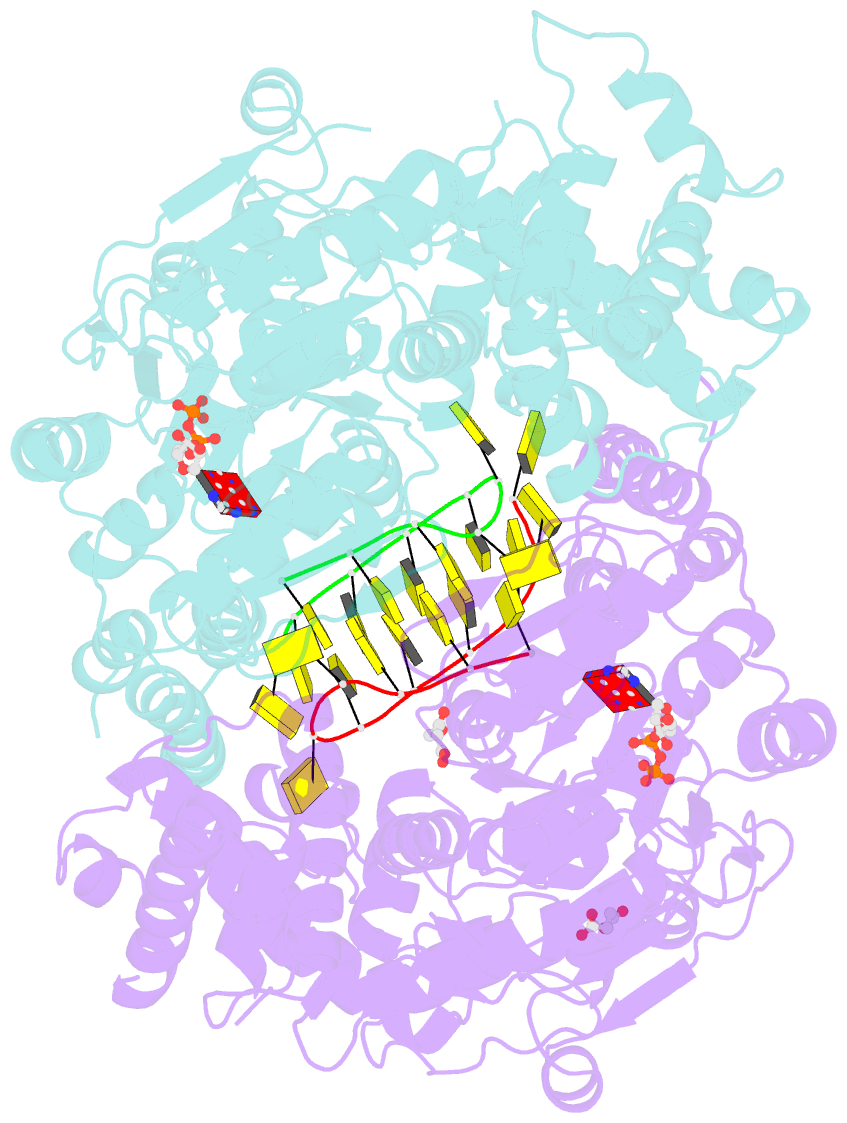
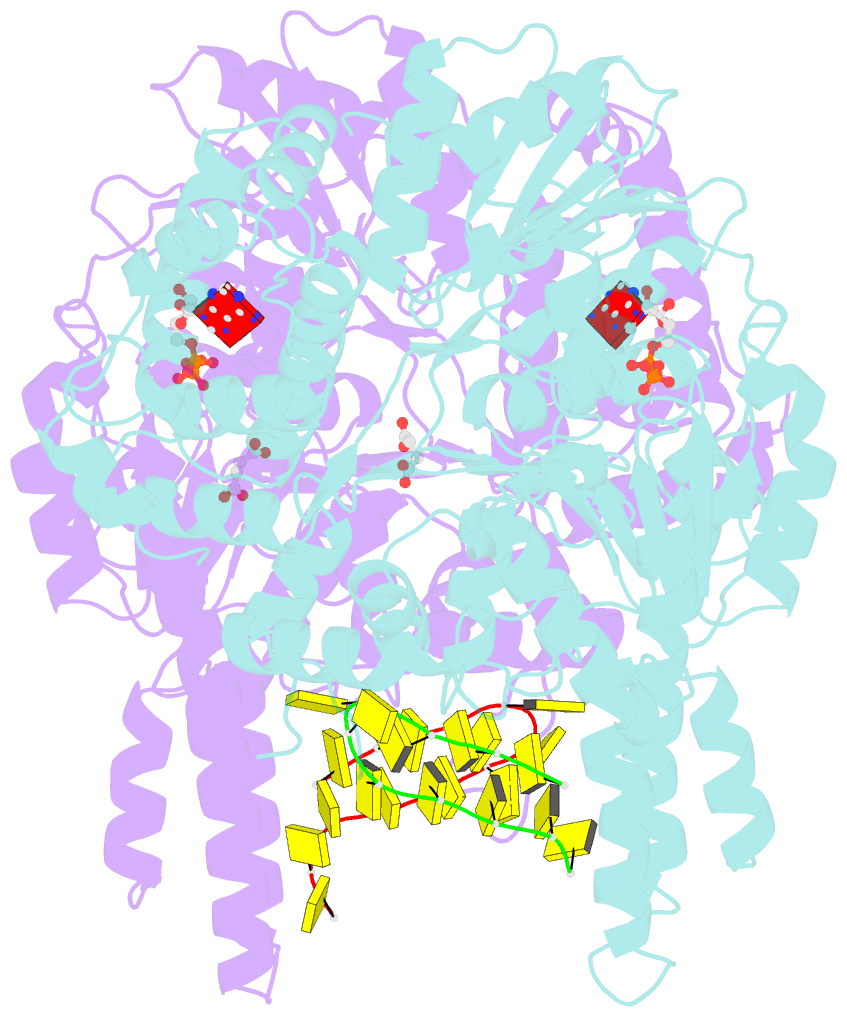
Summary information and primary citation
- PDB-id
- 8po8; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase
- Method
- X-ray (2.52 Å)
- Summary
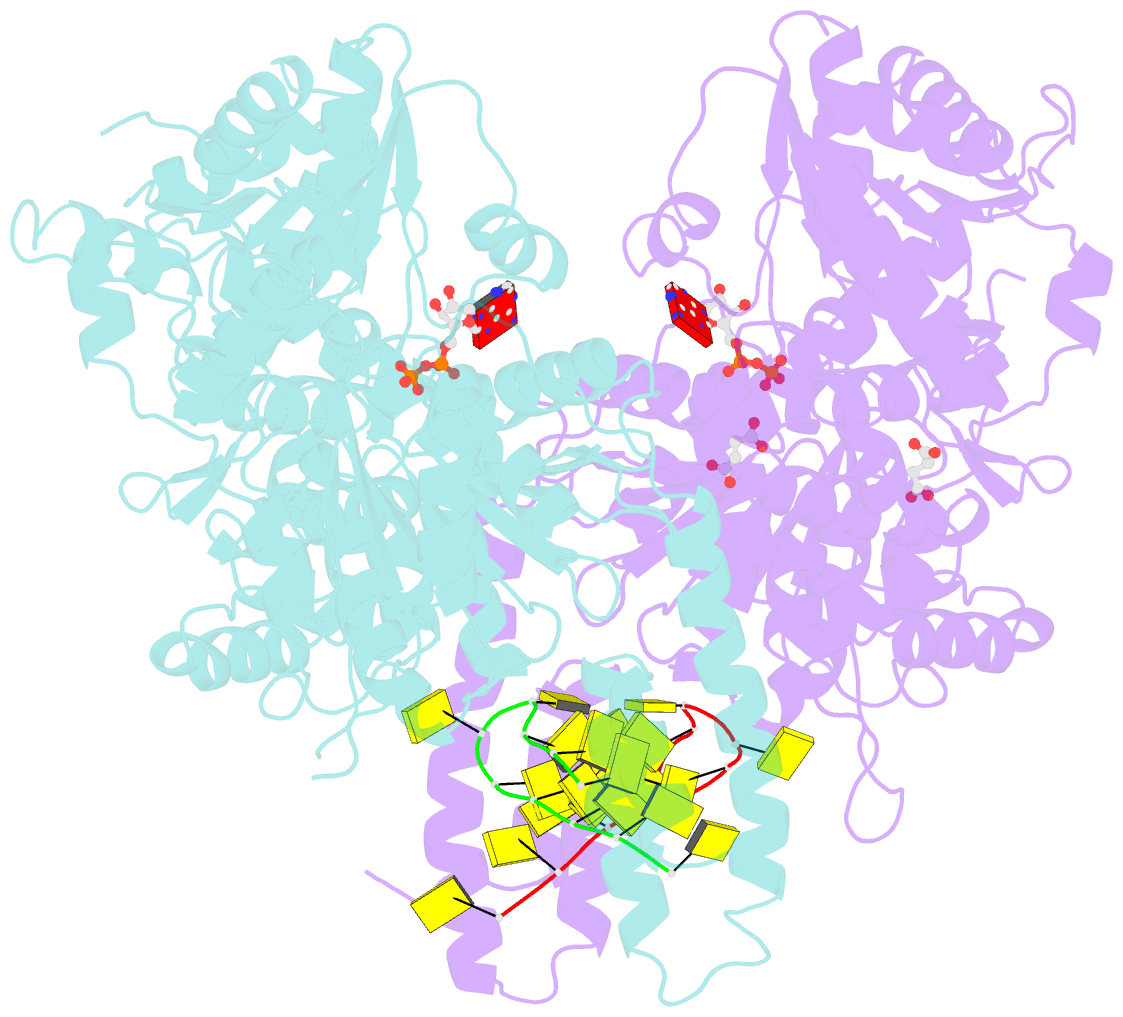
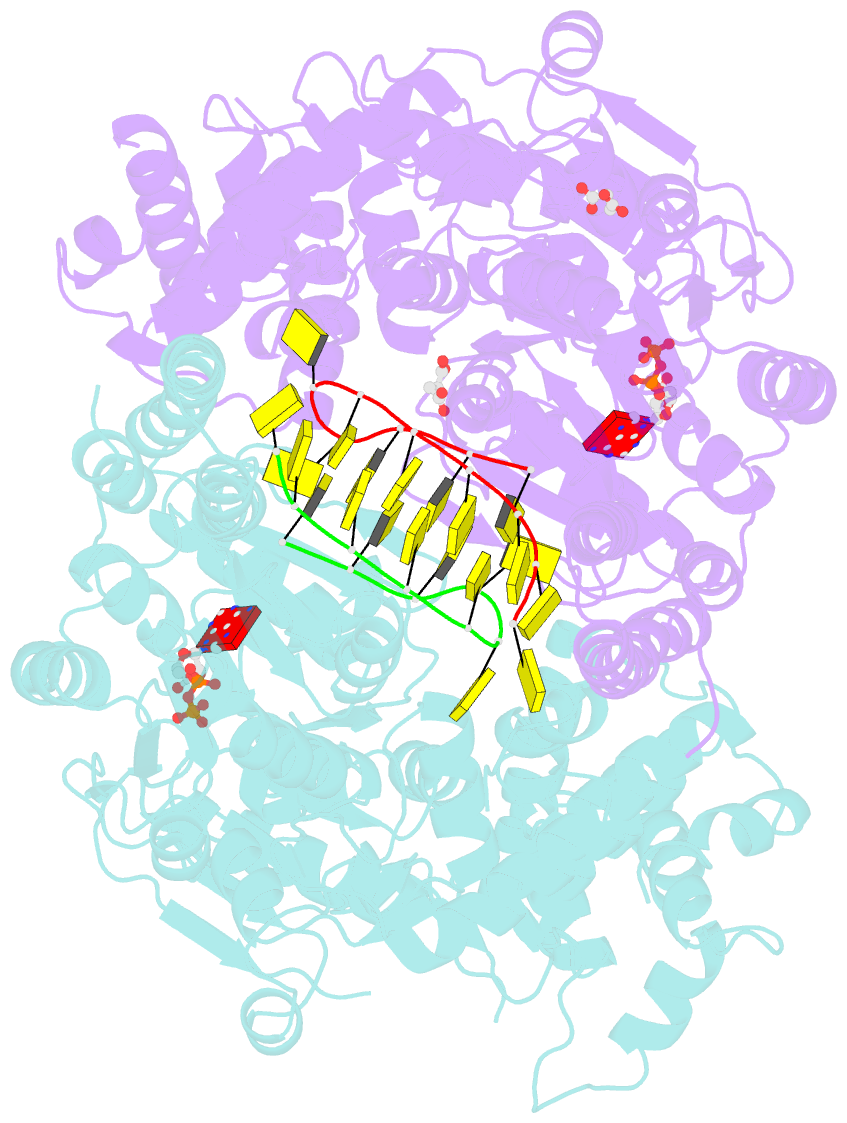
- Structure of escherichia coli hrpa in complex with adp and oligonucleotide poly(dc)11 forming an i-motif
- Reference
- Xin BG, Huang LY, Yuan LG, Liu NN, Li HH, Ai X, Lei DS, Hou XM, Rety S, Xi XG (2024): "Structural insights into the N-terminal APHB domain of HrpA: mediating canonical and i-motif recognition." Nucleic Acids Res., 52, 3406-3418. doi: 10.1093/nar/gkae138.
- Abstract
- RNA helicases function as versatile enzymes primarily responsible for remodeling RNA secondary structures and organizing ribonucleoprotein complexes. In our study, we conducted a systematic analysis of the helicase-related activities of Escherichia coli HrpA and presented the structures of both its apo form and its complex bound with both conventional and non-canonical DNAs. Our findings reveal that HrpA exhibits NTP hydrolysis activity and binds to ssDNA and ssRNA in distinct sequence-dependent manners. While the helicase core plays an essential role in unwinding RNA/RNA and RNA/DNA duplexes, the N-terminal extension in HrpA, consisting of three helices referred to as the APHB domain, is crucial for ssDNA binding and RNA/DNA duplex unwinding. Importantly, the APHB domain is implicated in binding to non-canonical DNA structures such as G-quadruplex and i-motif, and this report presents the first solved i-motif-helicase complex. This research not only provides comprehensive insights into the multifaceted roles of HrpA as an RNA helicase but also establishes a foundation for further investigations into the recognition and functional implications of i-motif DNA structures in various biological processes.