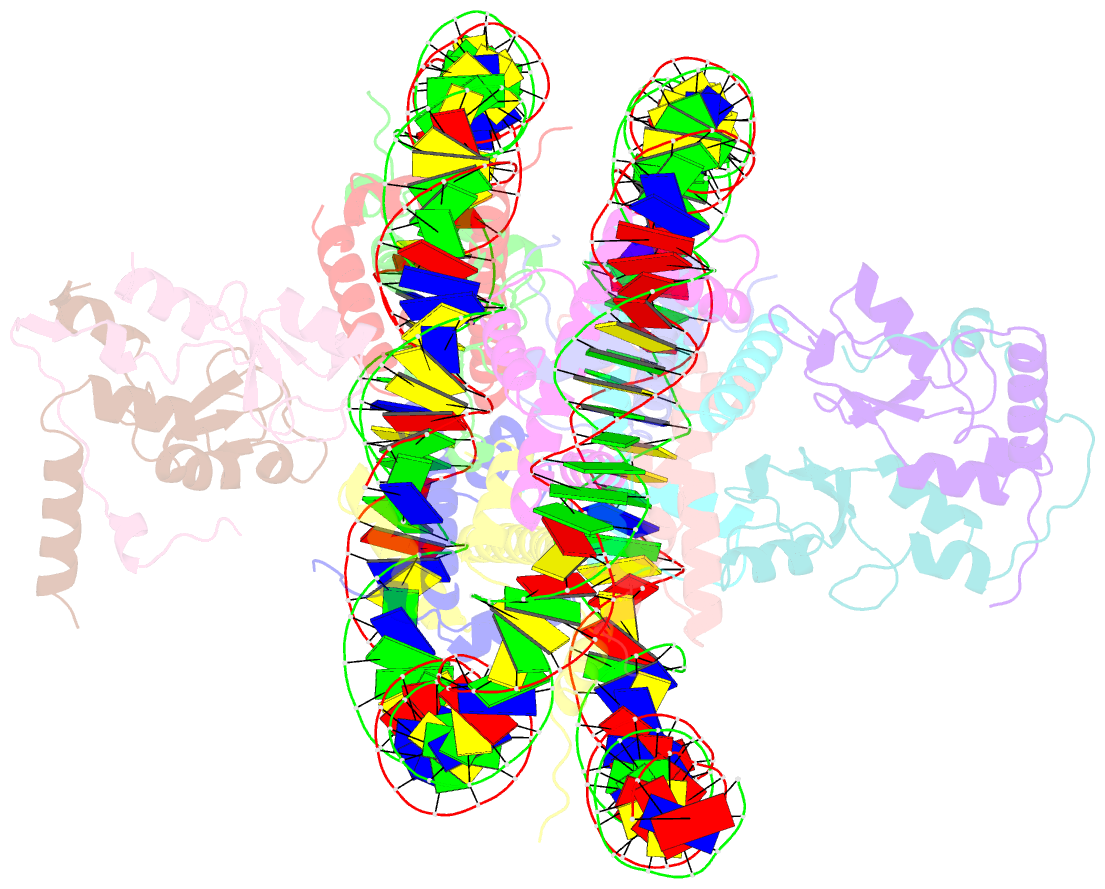
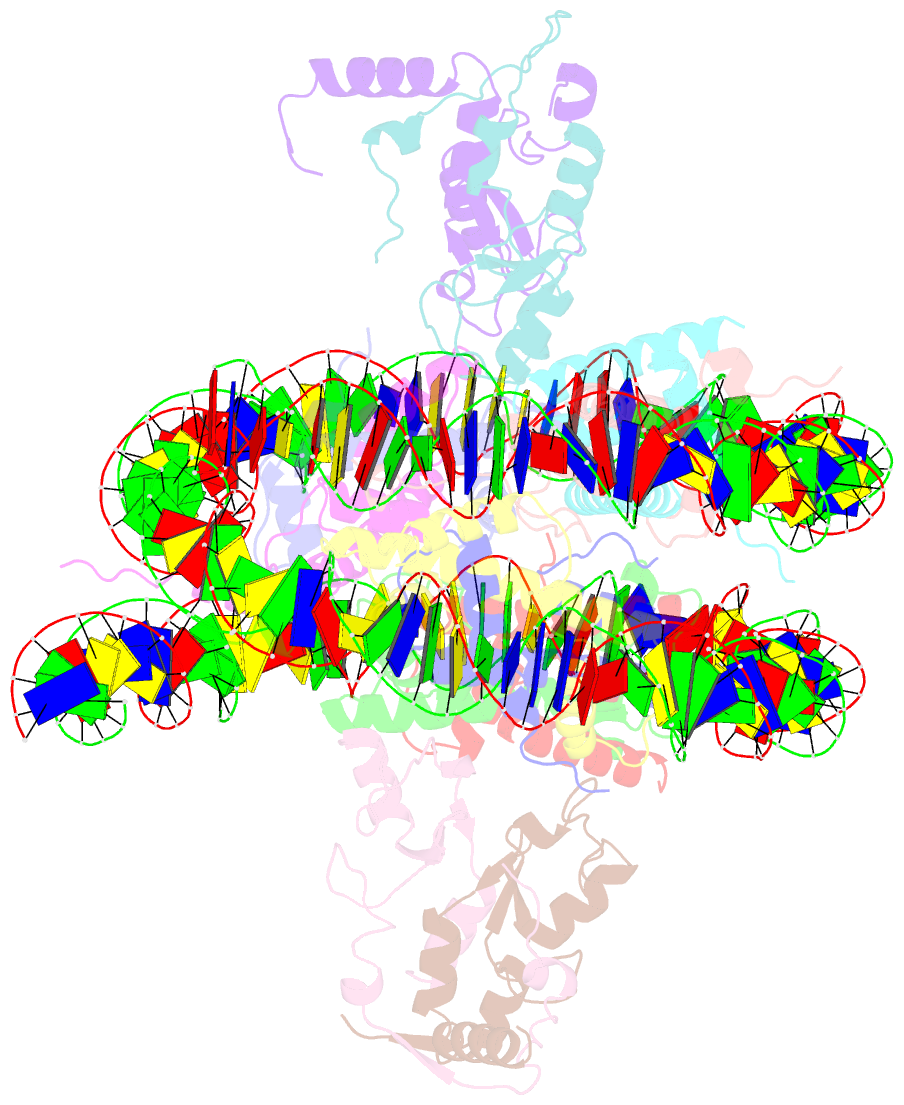
Summary information and primary citation
- PDB-id
- 8pp7; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- gene regulation
- Method
- cryo-EM (2.91 Å)
- Summary
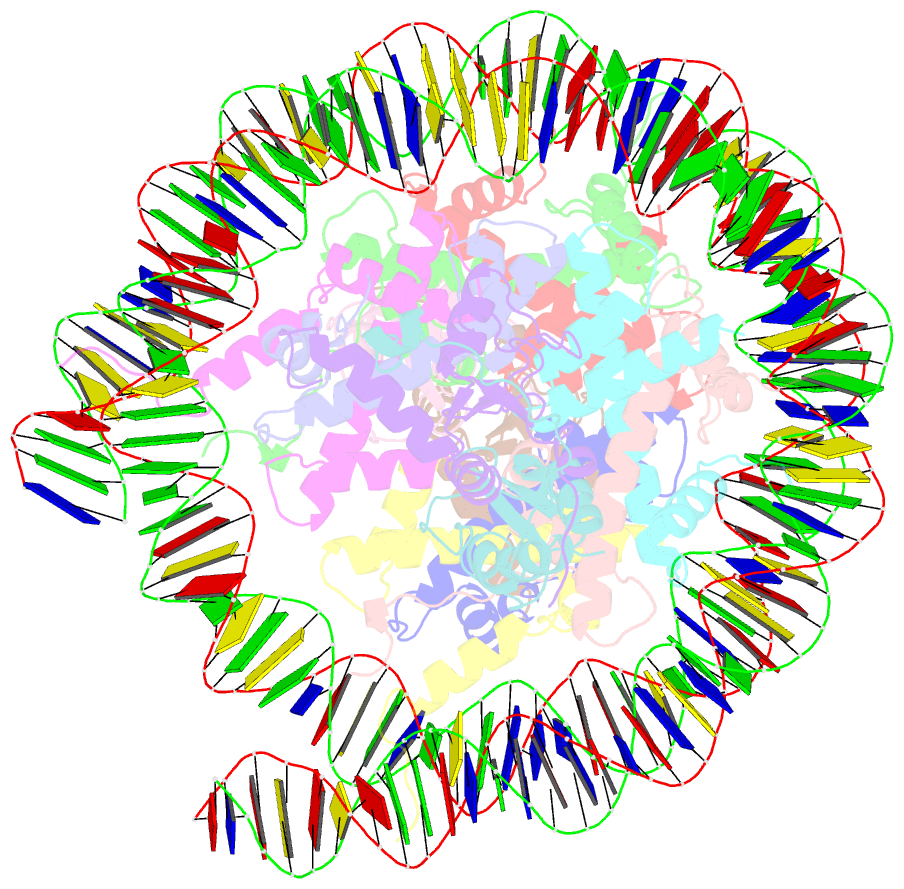
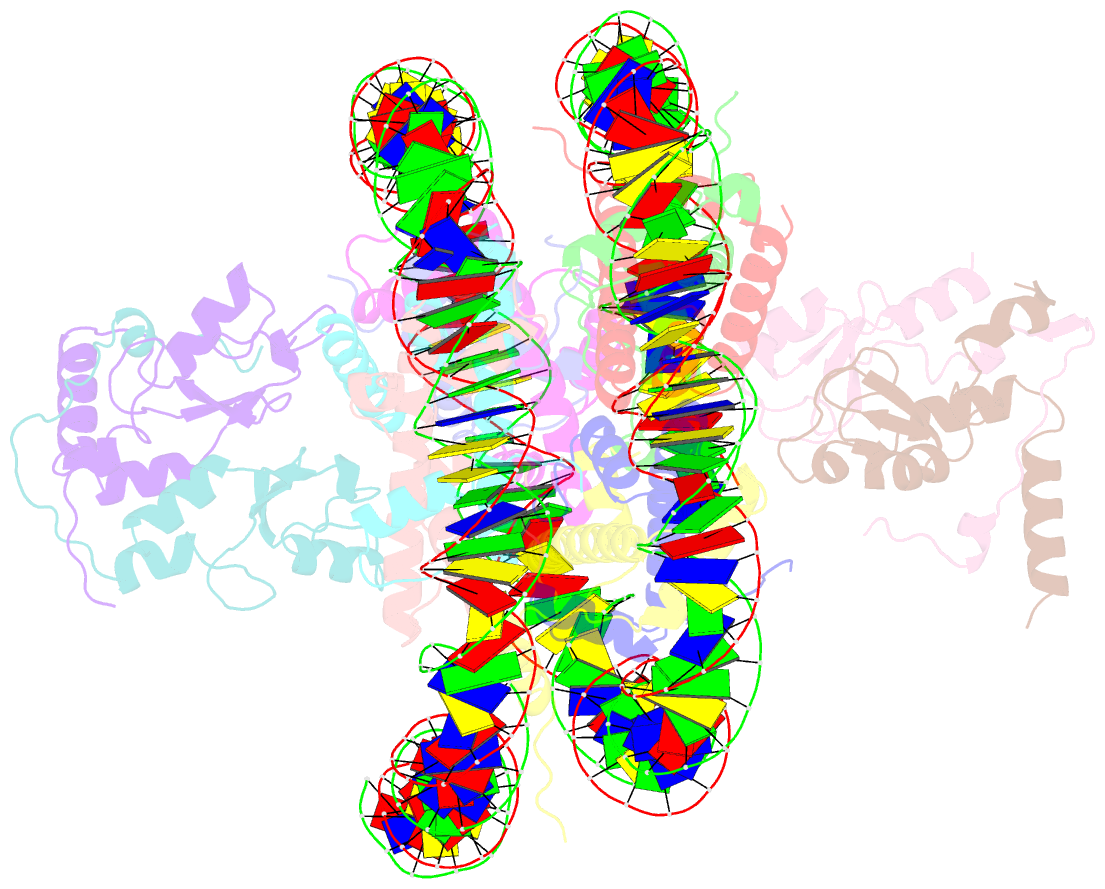
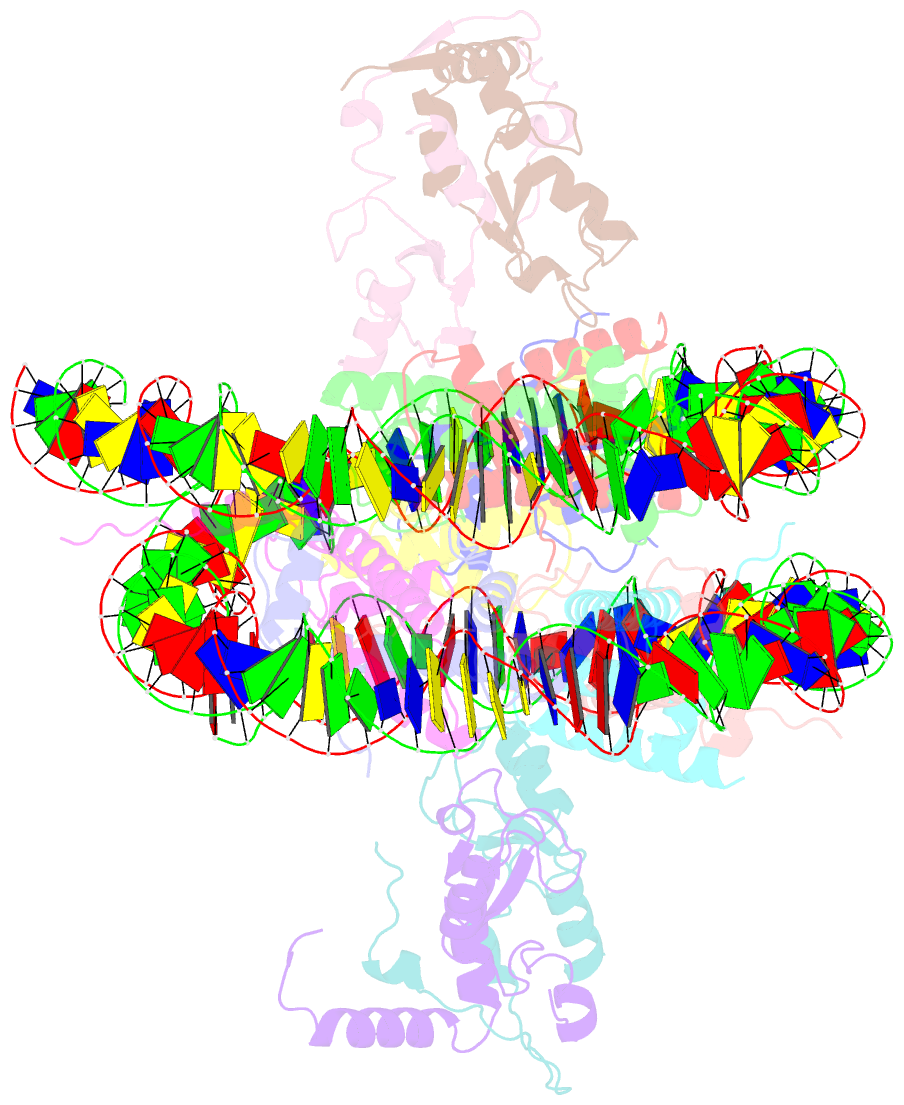
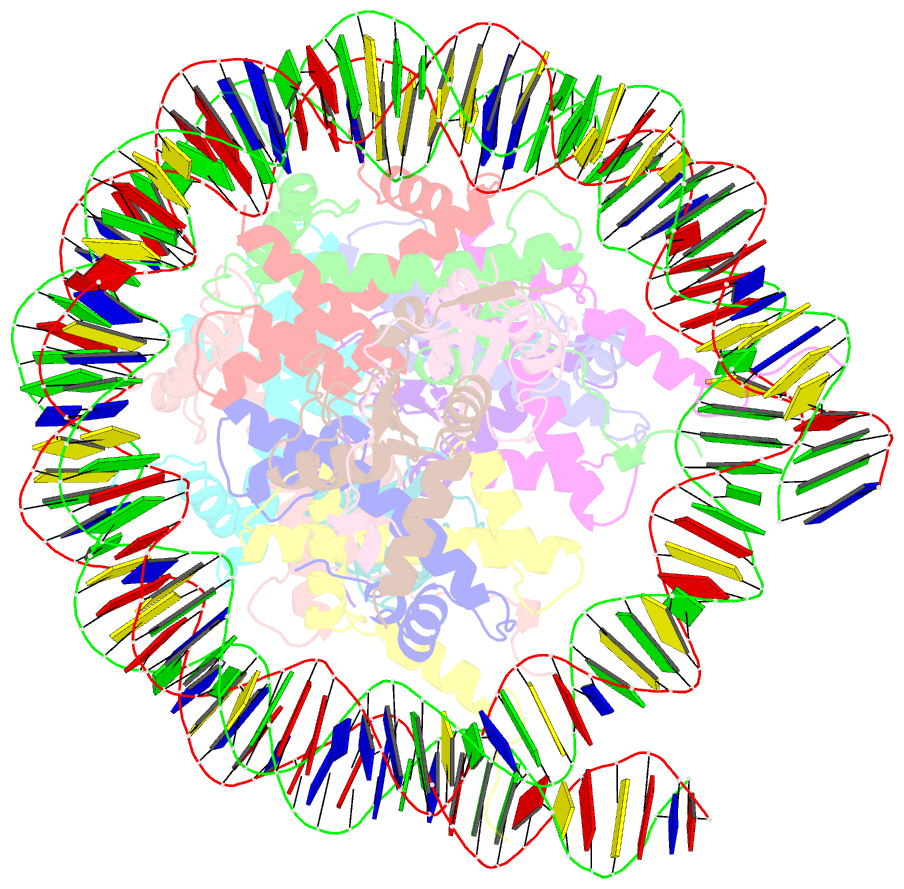
- Human rybp-prc1 bound to mononucleosome
- Reference
- Ciapponi M, Karlukova E, Schkolziger S, Benda C, Muller J (2024): "Structural basis of the histone ubiquitination read-write mechanism of RYBP-PRC1." Nat.Struct.Mol.Biol., 31, 1023-1027. doi: 10.1038/s41594-024-01258-x.
- Abstract
- Histone H2A monoubiquitination (H2Aub1) by the PRC1 subunit RING1B entails a positive feedback loop, mediated by the RING1B-interacting protein RYBP. We uncover that human RYBP-PRC1 binds unmodified nucleosomes via RING1B but H2Aub1-modified nucleosomes via RYBP. RYBP interactions with both ubiquitin and the nucleosome acidic patch create the high binding affinity that favors RYBP- over RING1B-directed PRC1 binding to H2Aub1-modified nucleosomes; this enables RING1B to monoubiquitinate H2A in neighboring unmodified nucleosomes.