Summary information and primary citation
- PDB-id
- 8pw0; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- X-ray (2.2 Å)
- Summary
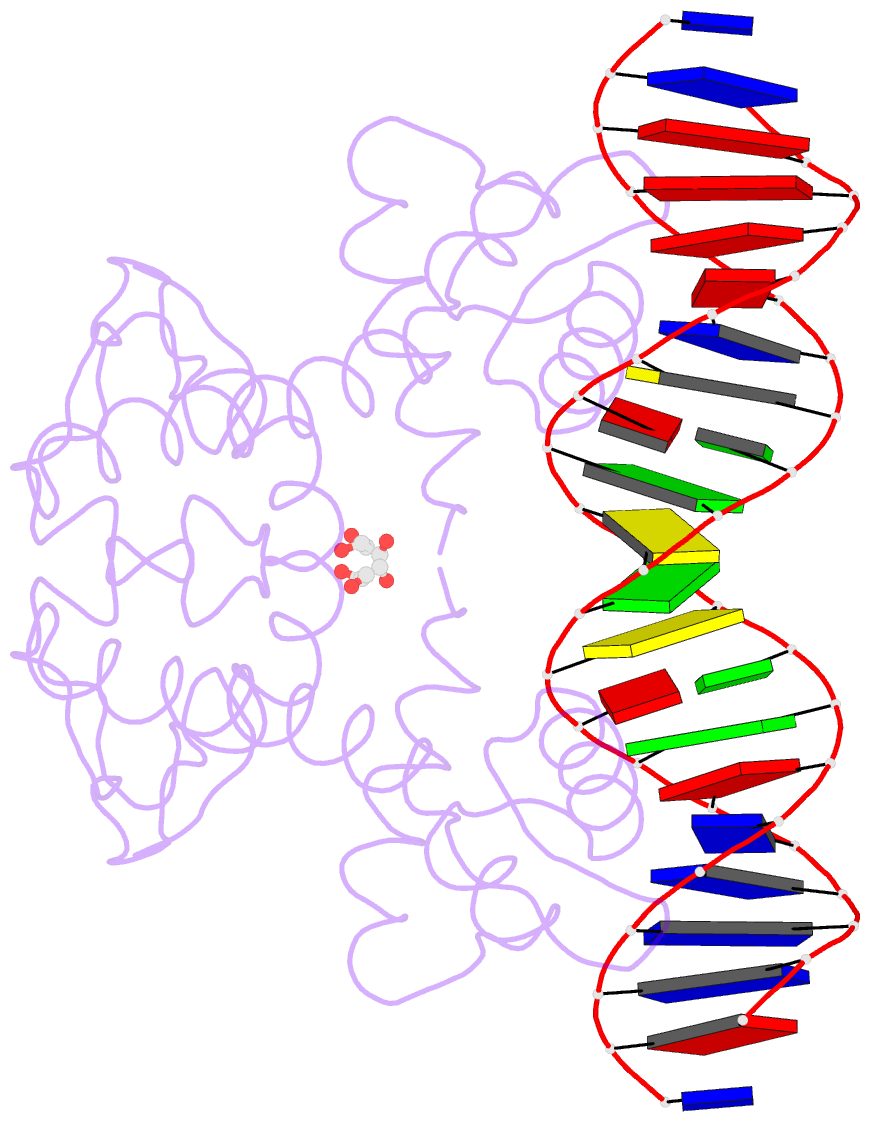
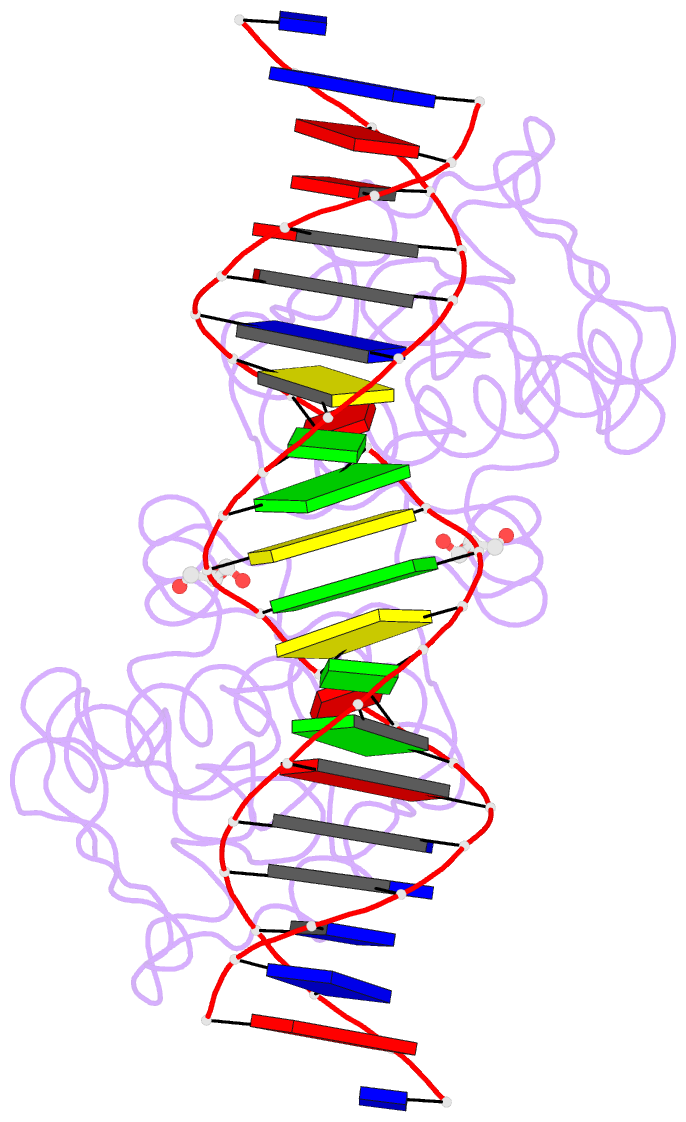
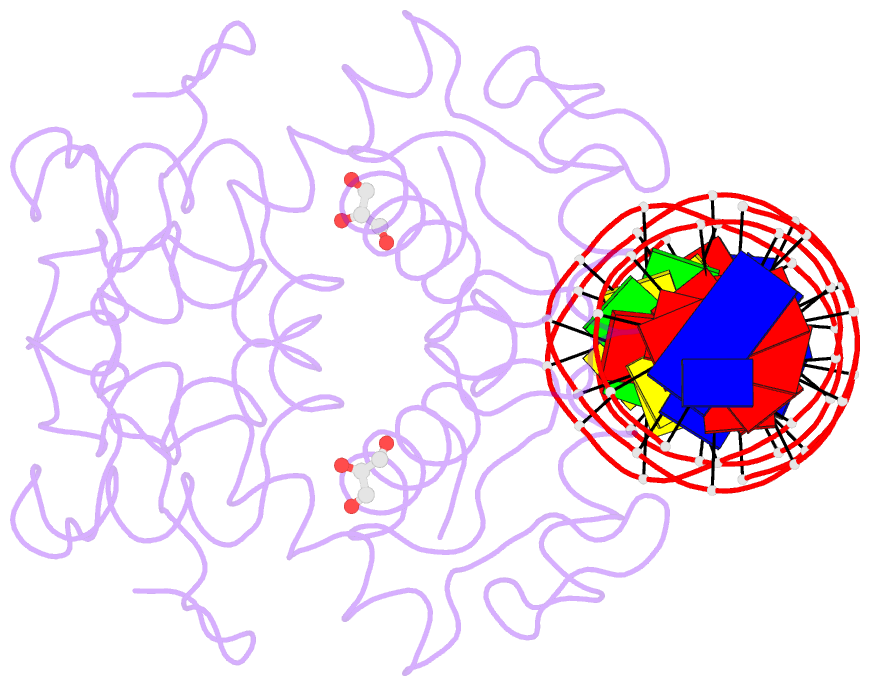
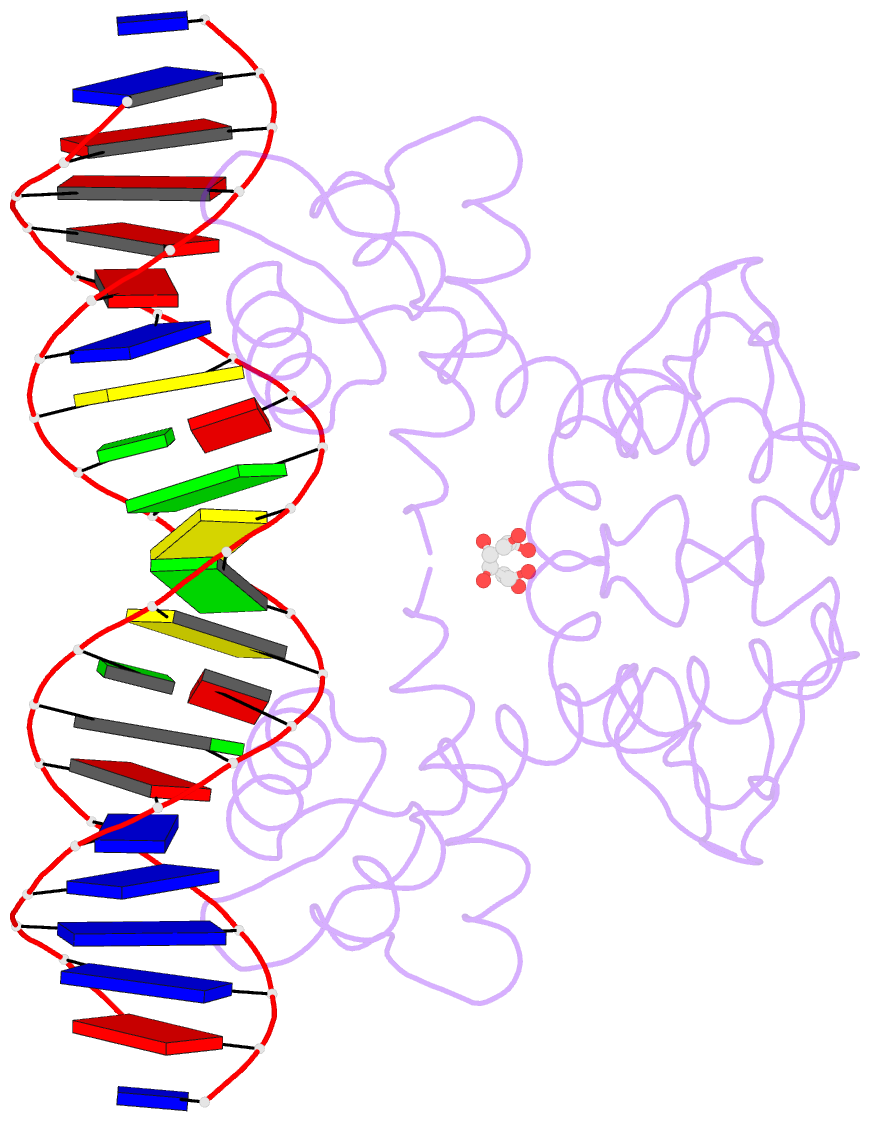
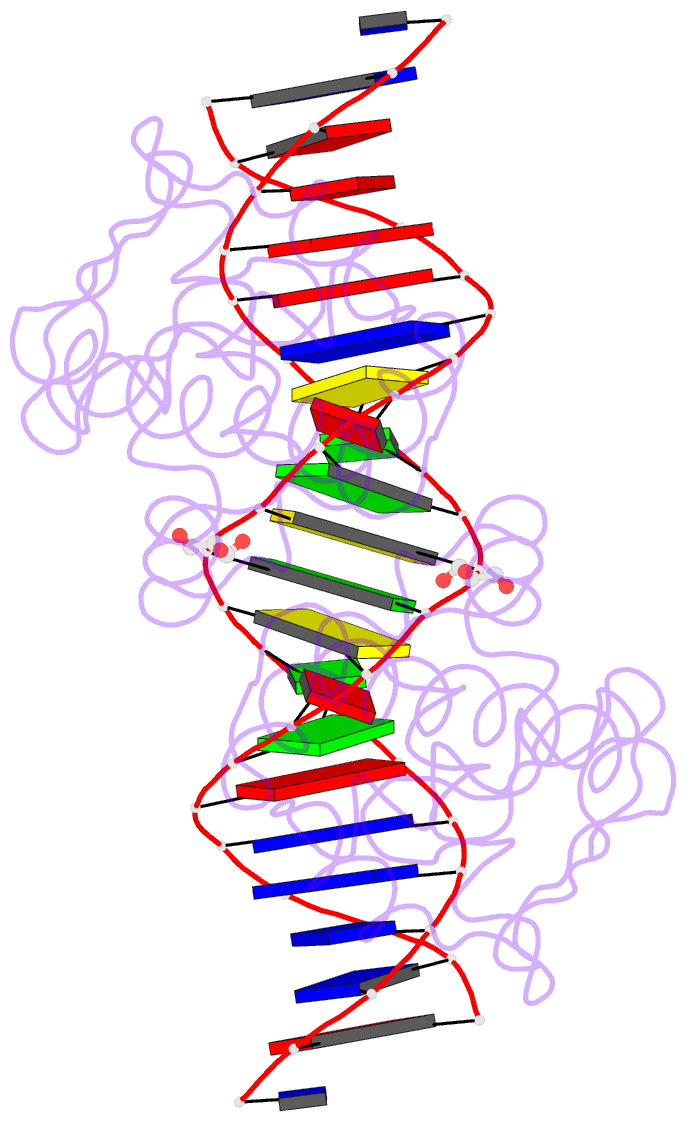
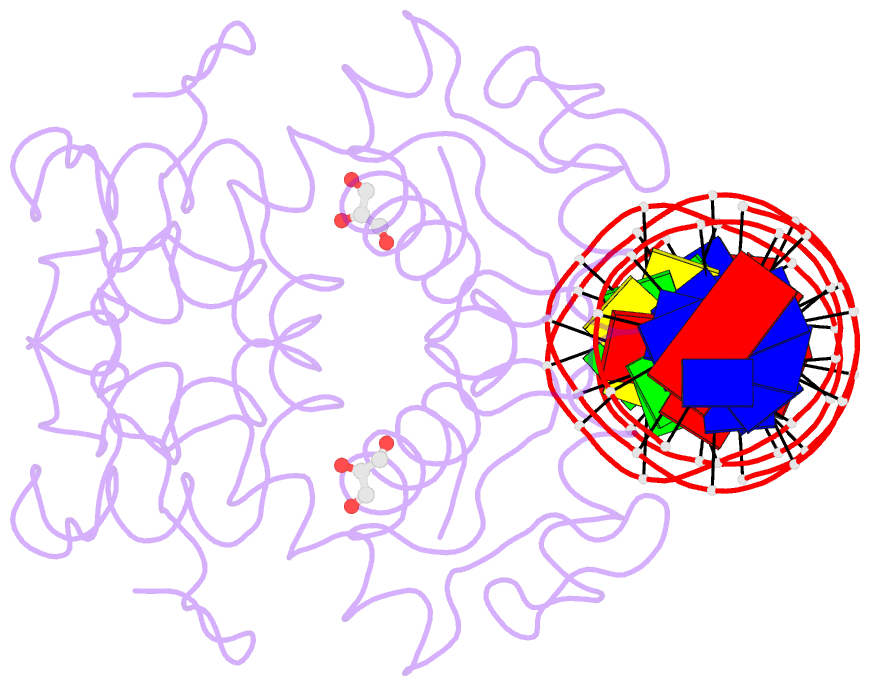
- Manganese-dependent transcriptional repressor dr2539 complexed with manganese and bound to dr1709 promotor
- Reference
- Mota C, Webster M, Saidi M, Kapp U, Zubieta C, Giachin G, Manso JA, de Sanctis D (2024): "Metal ion activation and DNA recognition by the Deinococcus radiodurans manganese sensor DR2539." Febs J., 291, 3384-3402. doi: 10.1111/febs.17140.
- Abstract
- The accumulation of manganese ions is crucial for scavenging reactive oxygen species and protecting the proteome of Deinococcus radiodurans (Dr). However, metal homeostasis still needs to be tightly regulated to avoid toxicity. DR2539, a dimeric transcription regulator, plays a key role in Dr manganese homeostasis. Despite comprising three well-conserved domains - a DNA-binding domain, a dimerisation domain, and an ancillary domain - the mechanisms underlying both, metal ion activation and DNA recognition remain elusive. In this study, we present biophysical analyses and the structure of the dimerisation and DNA-binding domains of DR2539 in its holo-form and in complex with the 21 base pair pseudo-palindromic repeat of the dr1709 promoter region, shedding light on these activation and recognition mechanisms. The dimer presents eight manganese binding sites that induce structural conformations essential for DNA binding. The analysis of the protein-DNA interfaces elucidates the significance of Tyr59 and helix α3 sequence in the interaction with the DNA. Finally, the structure in solution as determined by small-angle X-ray scattering experiments and supported by AlphaFold modeling provides a model illustrating the conformational changes induced upon metal binding.