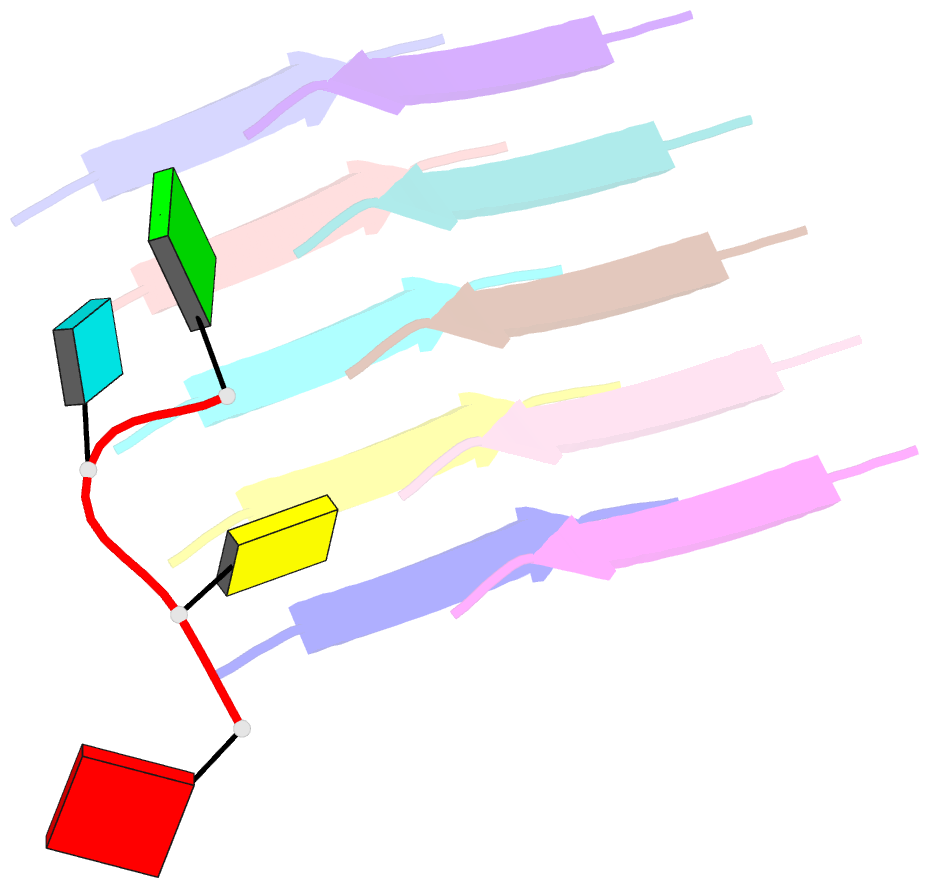
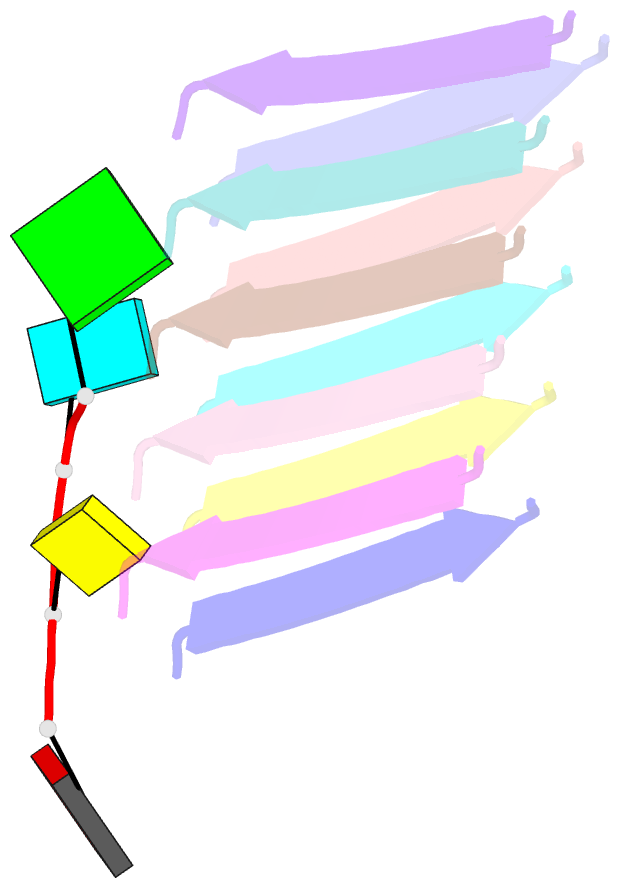
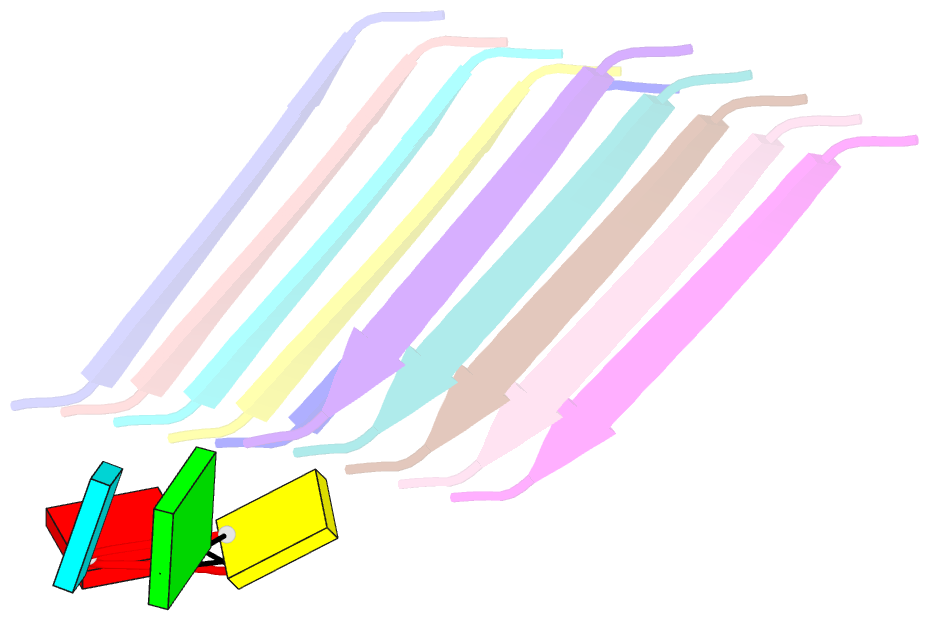
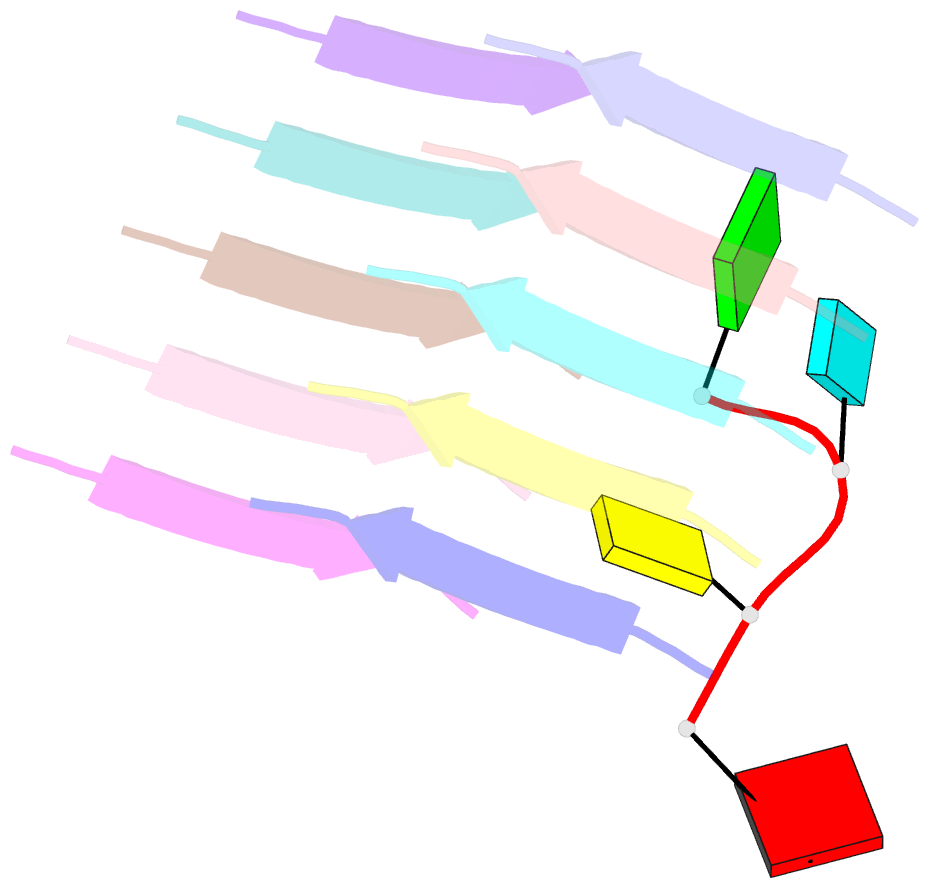
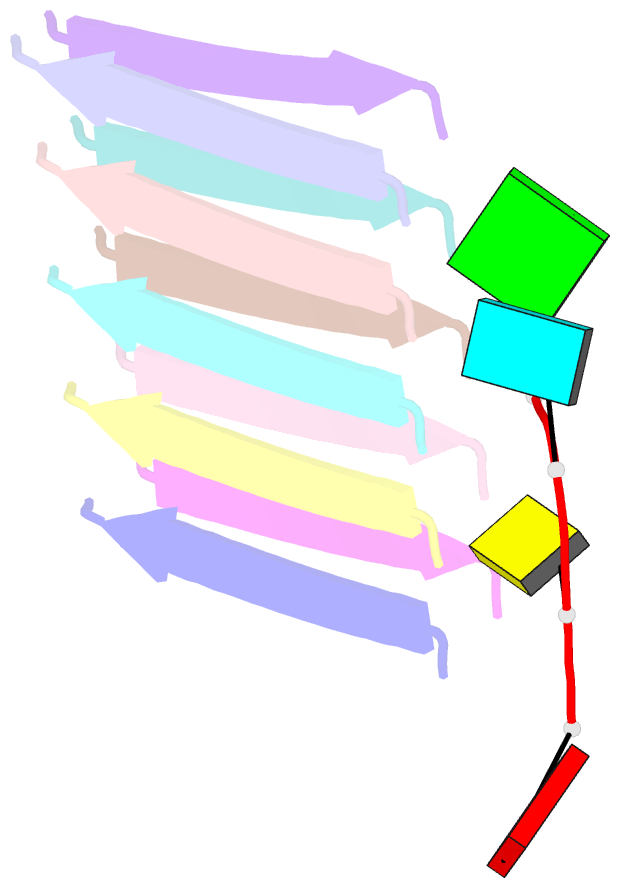
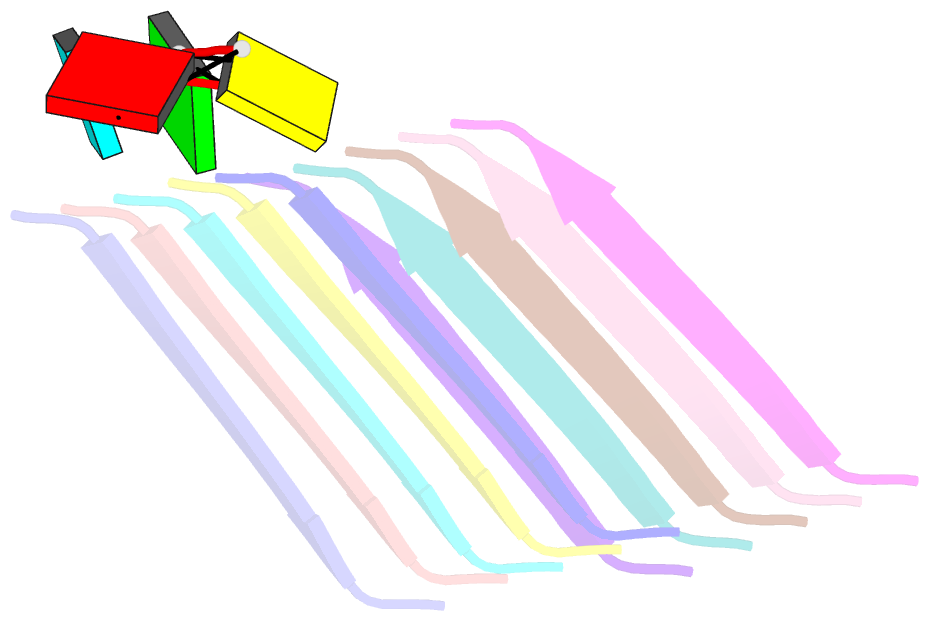
Summary information and primary citation
- PDB-id
- 8pxs; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- unknown function
- Method
- NMR
- Summary
- Short RNA binding to peptide amyloids
- Reference
- Rout SK, Cadalbert R, Schroder N, Wang J, Zehnder J, Gampp O, Wiegand T, Guntert P, Klingler D, Kreutz C, Knorlein A, Hall J, Greenwald J, Riek R (2023): "An Analysis of Nucleotide-Amyloid Interactions Reveals Selective Binding to Codon-Sized RNA." J.Am.Chem.Soc., 145, 21915-21924. doi: 10.1021/jacs.3c06287.
- Abstract
- Interactions between RNA and proteins are the cornerstone of many important biological processes from transcription and translation to gene regulation, yet little is known about the ancient origin of said interactions. We hypothesized that peptide amyloids played a role in the origin of life and that their repetitive structure lends itself to building interfaces with other polymers through avidity. Here, we report that short RNA with a minimum length of three nucleotides binds in a sequence-dependent manner to peptide amyloids. The 3'-5' linked RNA backbone appears to be well-suited to support these interactions, with the phosphodiester backbone and nucleobases both contributing to the affinity. Sequence-specific RNA-peptide interactions of the kind identified here may provide a path to understanding one of the great mysteries rooted in the origin of life: the origin of the genetic code.