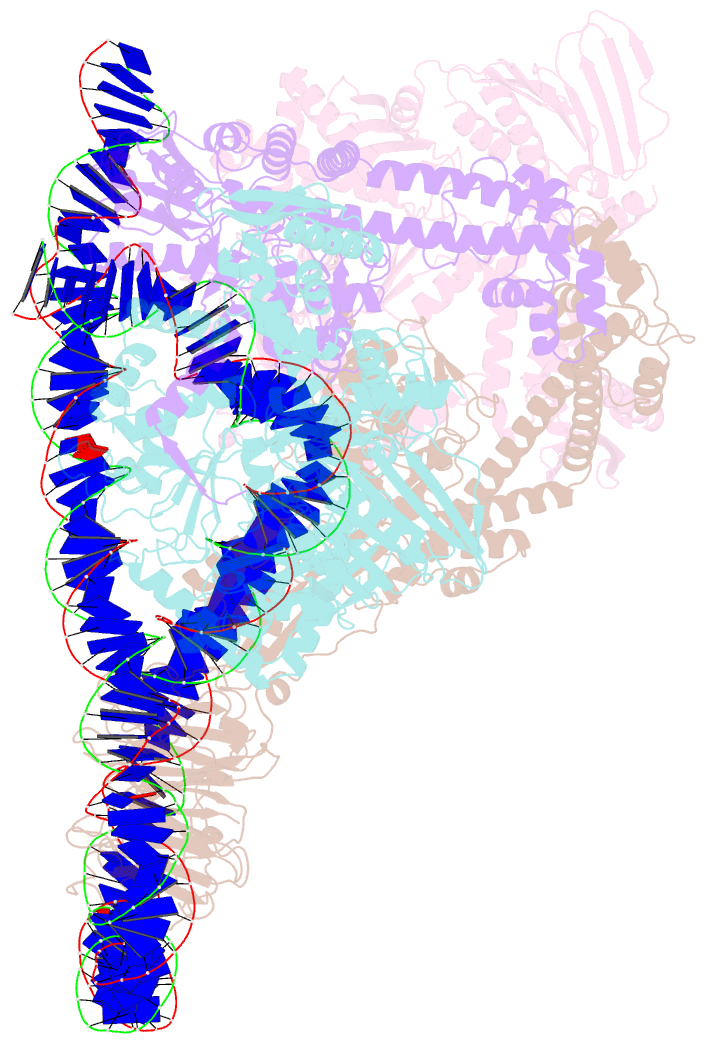
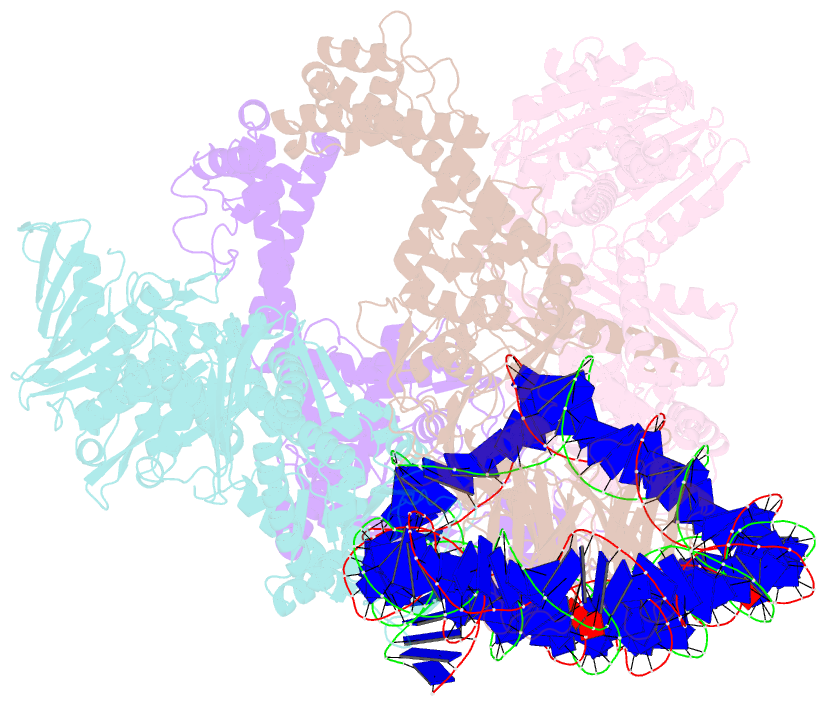
Summary information and primary citation
- PDB-id
- 8qdx; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- isomerase
- Method
- cryo-EM (3.0 Å)
- Summary
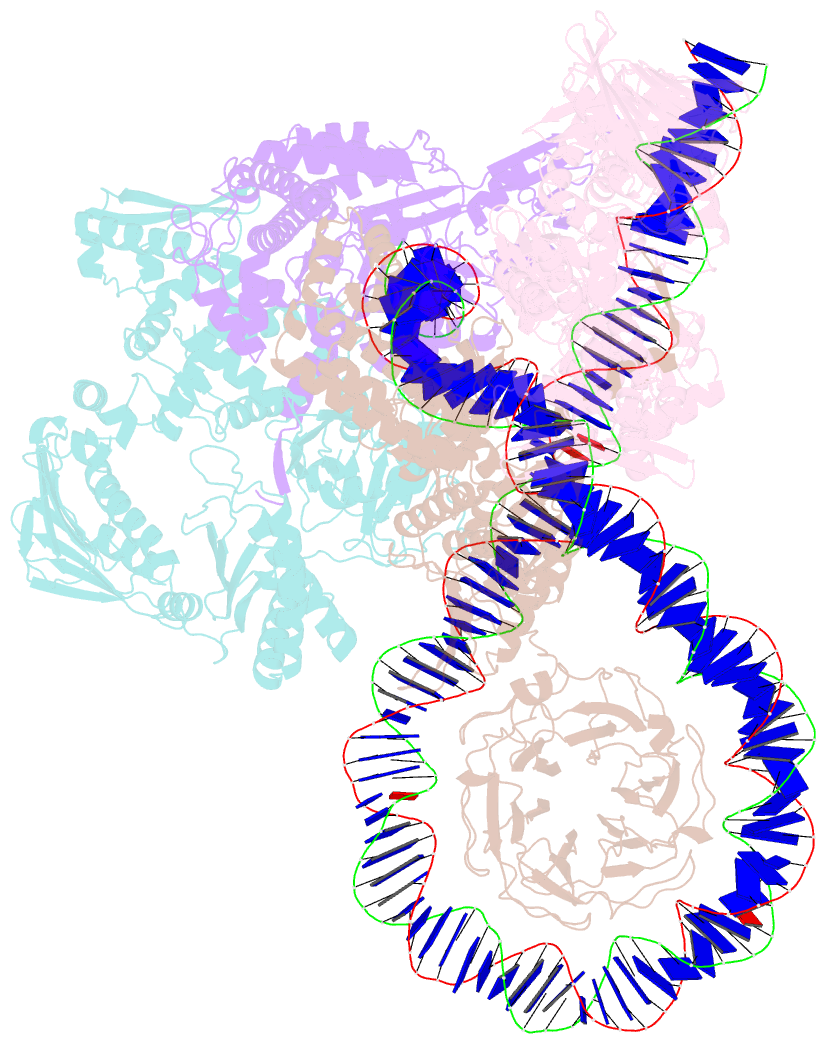
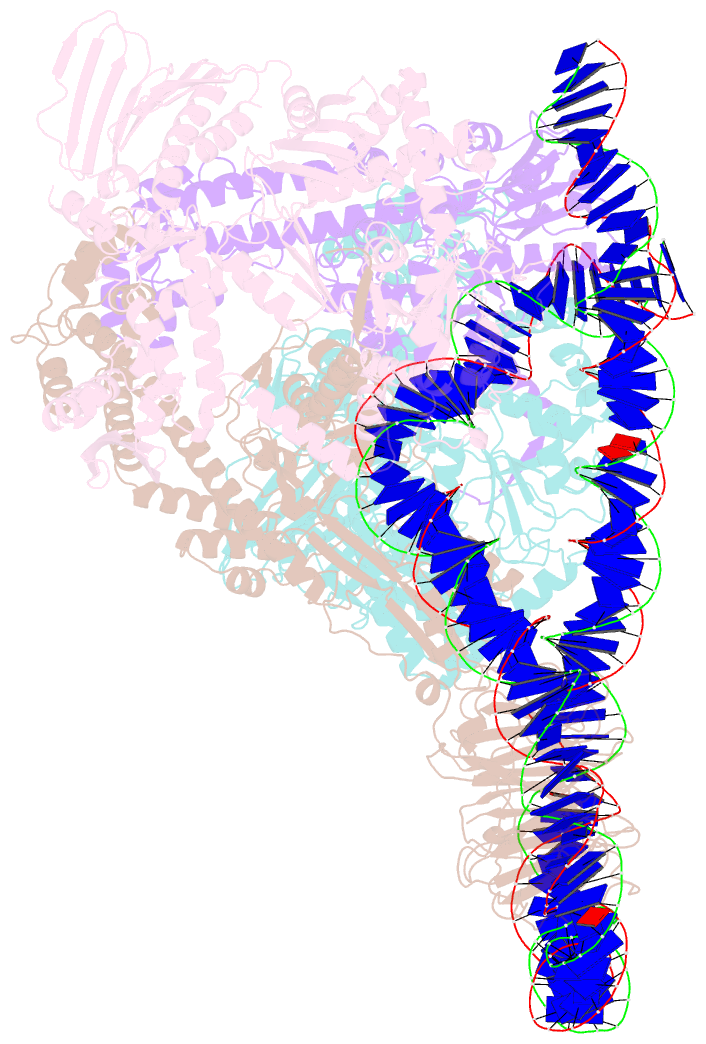
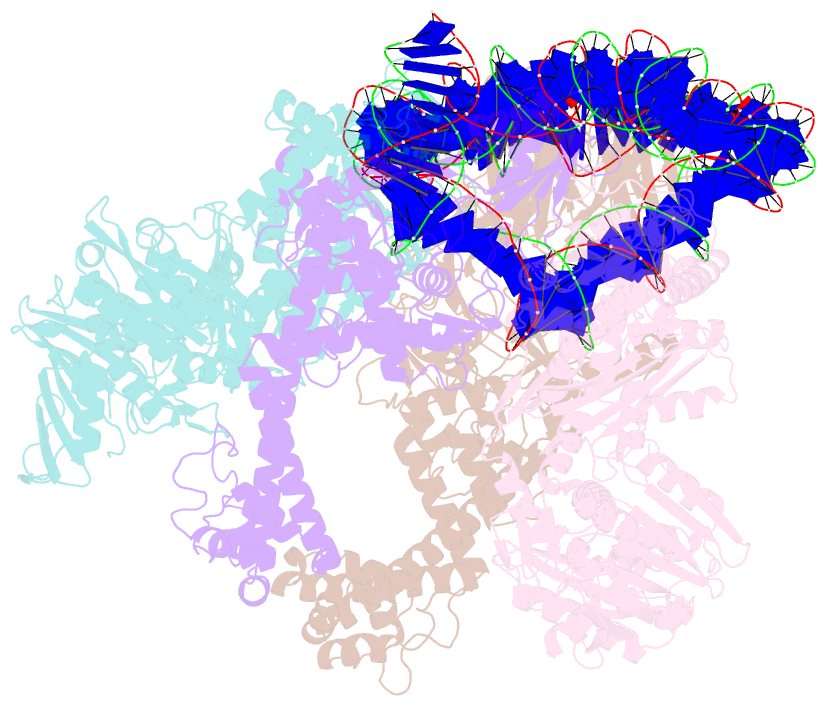
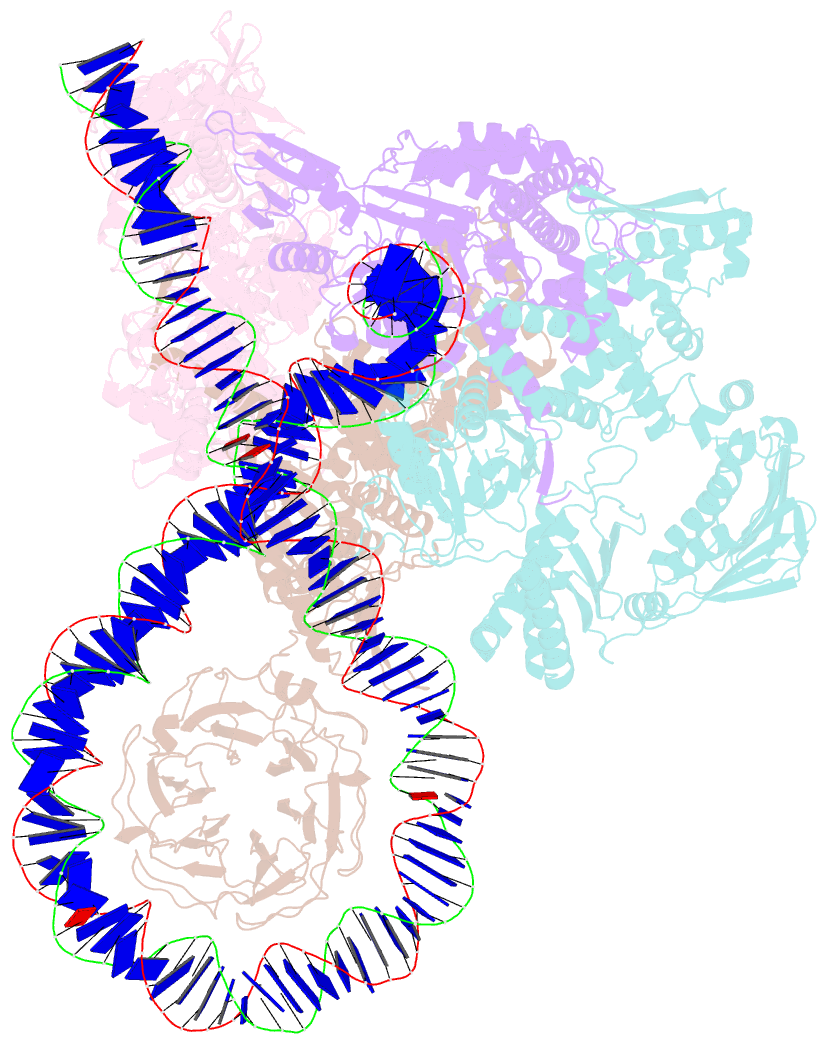
- E. coli DNA gyrase bound to a DNA crossover
- Reference
- Vayssieres M, Marechal N, Yun L, Lopez Duran B, Murugasamy NK, Fogg JM, Zechiedrich L, Nadal M, Lamour V (2024): "Structural basis of DNA crossover capture by Escherichia coli DNA gyrase." Science, 384, 227-232. doi: 10.1126/science.adl5899.
- Abstract
- DNA supercoiling must be precisely regulated by topoisomerases to prevent DNA entanglement. The interaction of type IIA DNA topoisomerases with two DNA molecules, enabling the transport of one duplex through the transient double-stranded break of the other, remains elusive owing to structures derived solely from single linear duplex DNAs lacking topological constraints. Using cryo-electron microscopy, we solved the structure of Escherichia coli DNA gyrase bound to a negatively supercoiled minicircle DNA. We show how DNA gyrase captures a DNA crossover, revealing both conserved molecular grooves that accommodate the DNA helices. Together with molecular tweezer experiments, the structure shows that the DNA crossover is of positive chirality, reconciling the binding step of gyrase-mediated DNA relaxation and supercoiling in a single structure.