Summary information and primary citation
- PDB-id
- 8siy; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- replication
- Method
- cryo-EM (2.9 Å)
- Summary
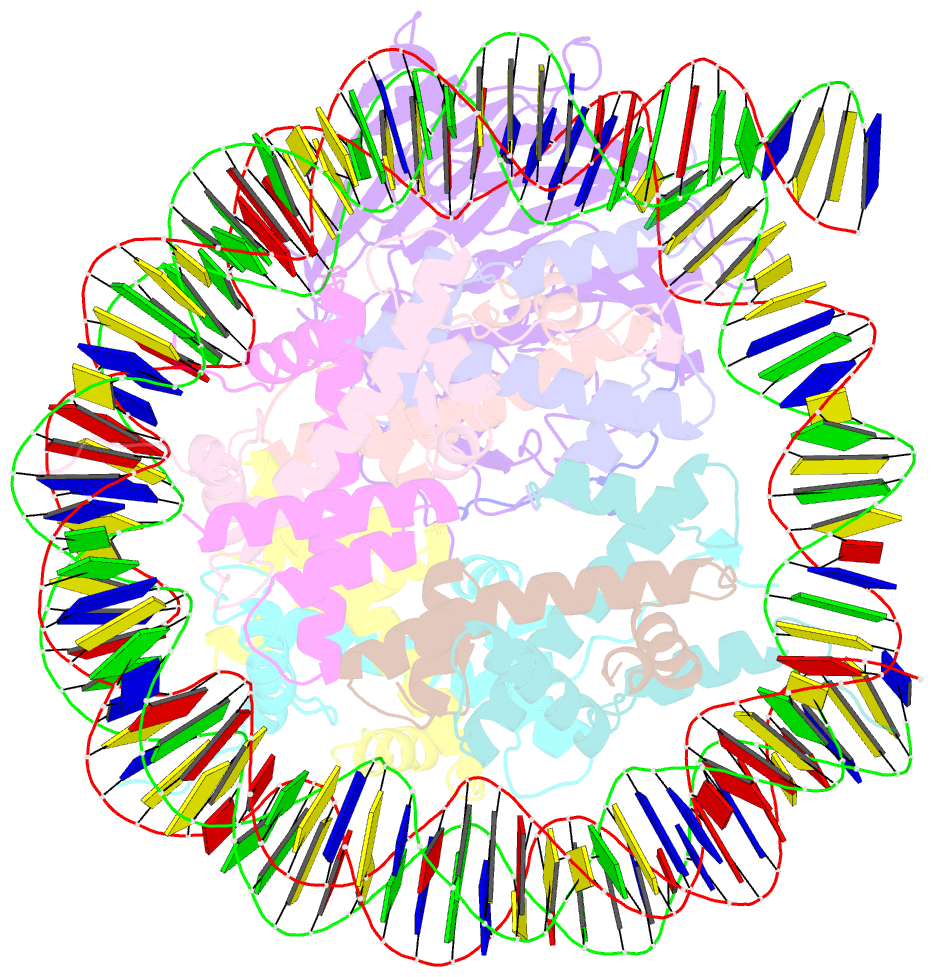
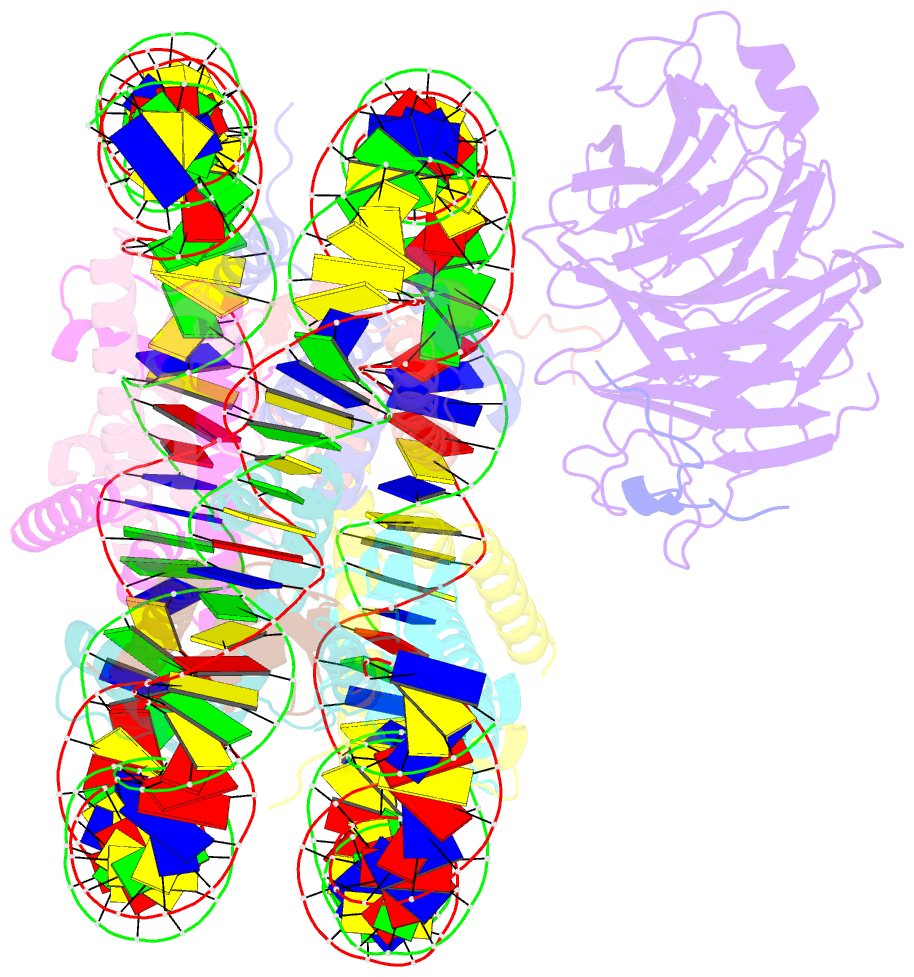
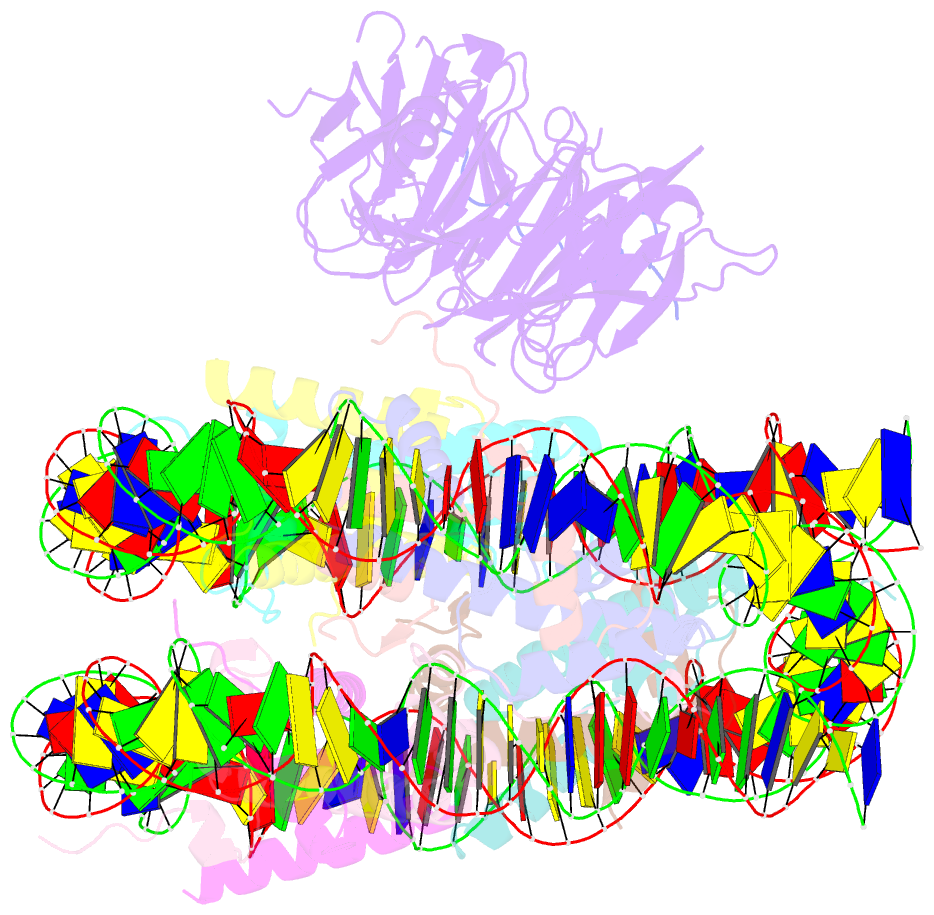
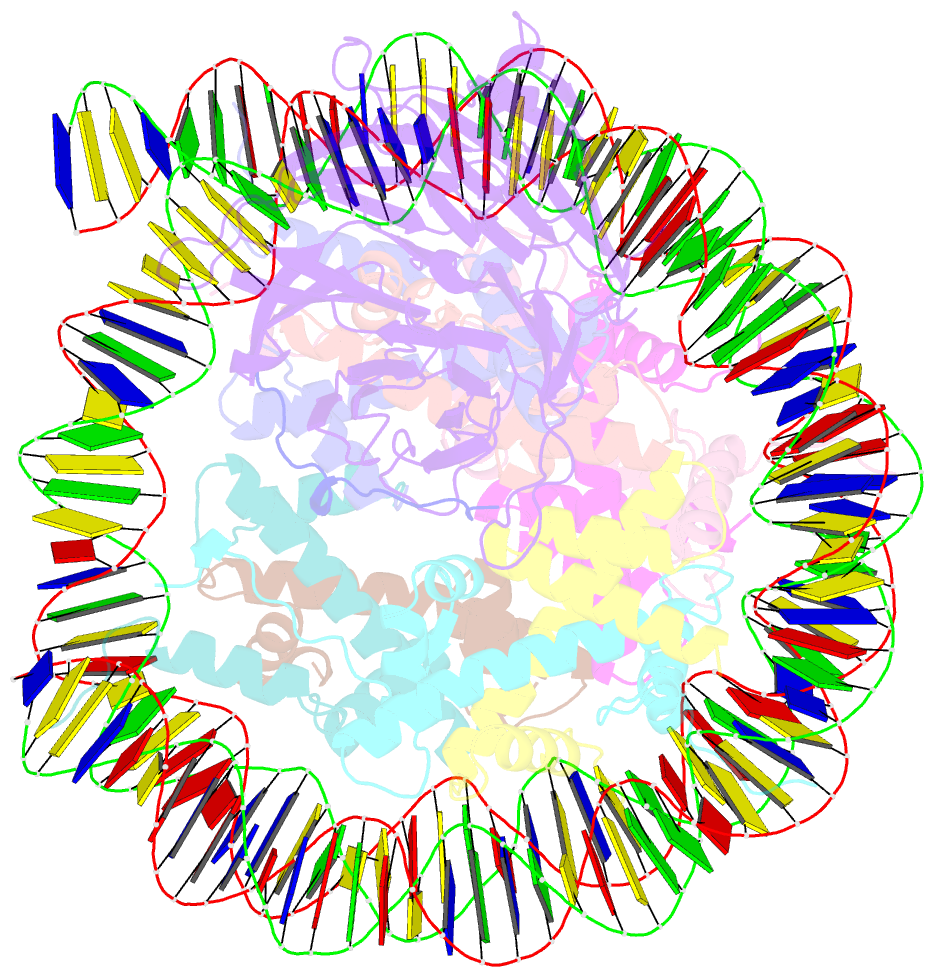
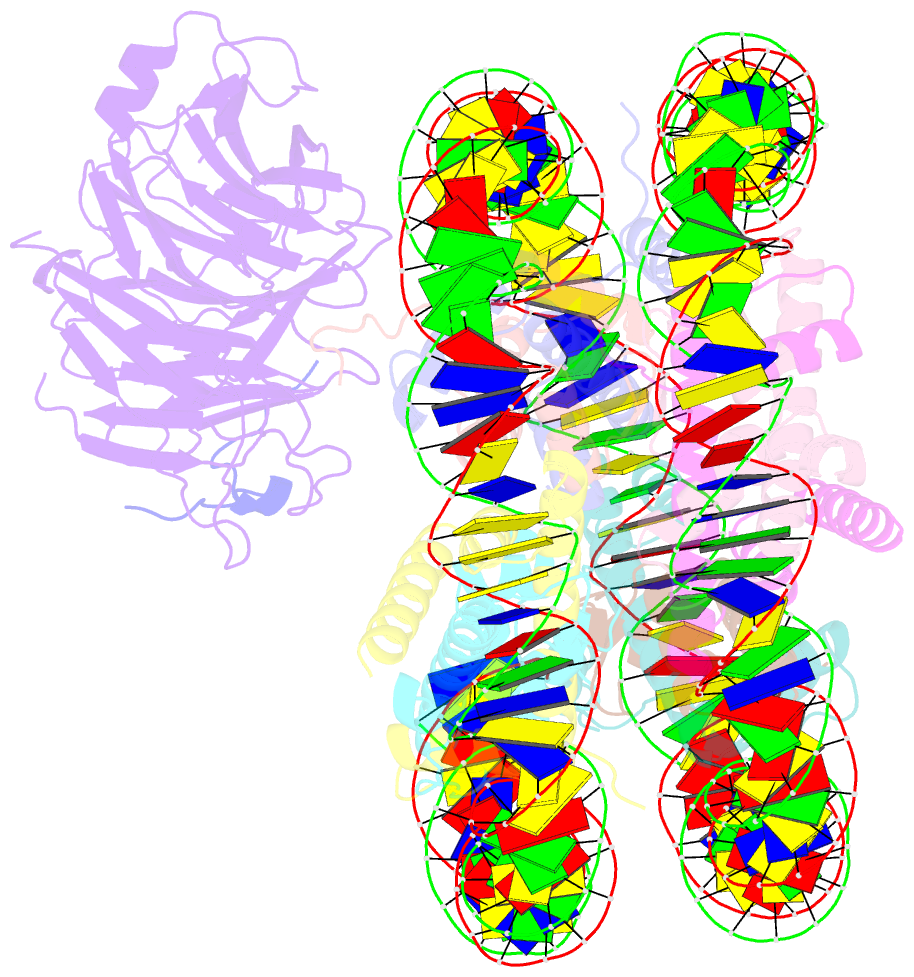
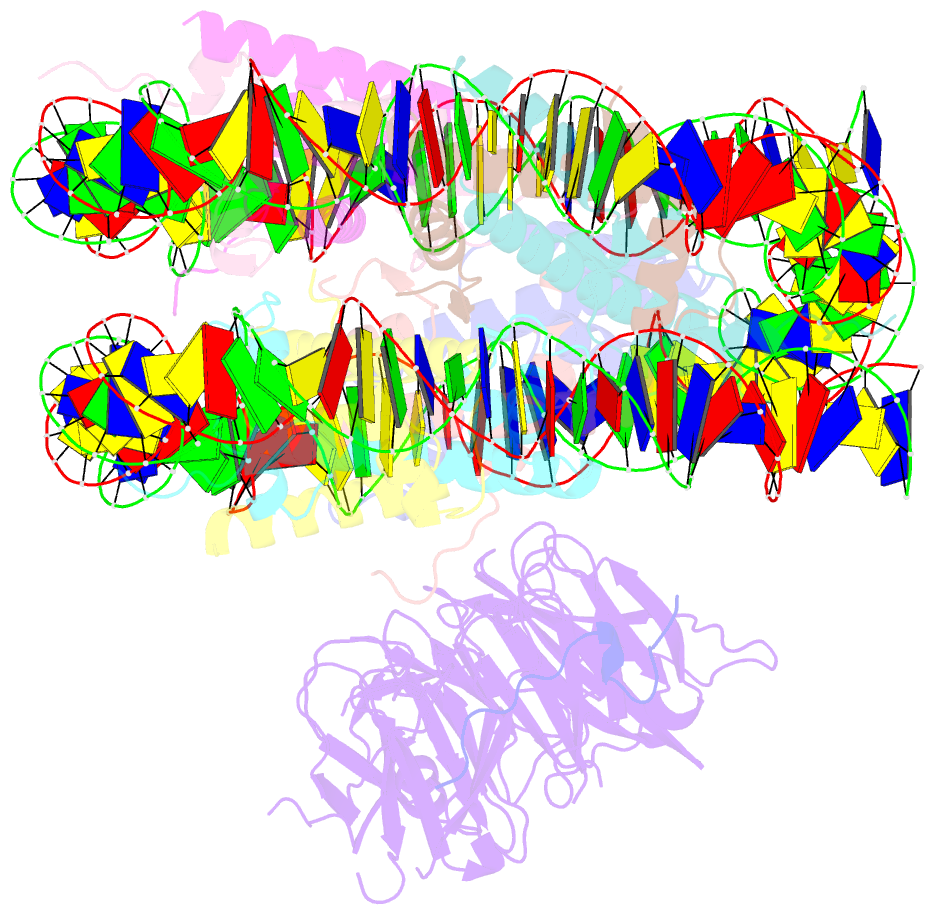
- Origin recognition complex associated (orca) protein bound to h4k20me3-nucleosome
- Reference
- Sahu S, Ekundayo BE, Kumar A, Bleichert F (2023): "A dual role for the chromatin reader ORCA/LRWD1 in targeting the origin recognition complex to chromatin." Embo J., 42, e114654. doi: 10.15252/embj.2023114654.
- Abstract
- Eukaryotic cells use chromatin marks to regulate the initiation of DNA replication. The origin recognition complex (ORC)-associated protein ORCA plays a critical role in heterochromatin replication in mammalian cells by recruiting the initiator ORC, but the underlying mechanisms remain unclear. Here, we report crystal and cryo-electron microscopy structures of ORCA in complex with ORC's Orc2 subunit and nucleosomes, establishing that ORCA orchestrates ternary complex assembly by simultaneously recognizing a highly conserved peptide sequence in Orc2, nucleosomal DNA, and repressive histone trimethylation marks through an aromatic cage. Unexpectedly, binding of ORCA to nucleosomes prevents chromatin array compaction in a manner that relies on H4K20 trimethylation, a histone modification critical for heterochromatin replication. We further show that ORCA is necessary and sufficient to specifically recruit ORC into chromatin condensates marked by H4K20 trimethylation, providing a paradigm for studying replication initiation in specific chromatin contexts. Collectively, our findings support a model in which ORCA not only serves as a platform for ORC recruitment to nucleosomes bearing specific histone marks but also helps establish a local chromatin environment conducive to subsequent MCM2-7 loading.