Summary information and primary citation
- PDB-id
- 8sna; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- cryo-EM (4.0 Å)
- Summary
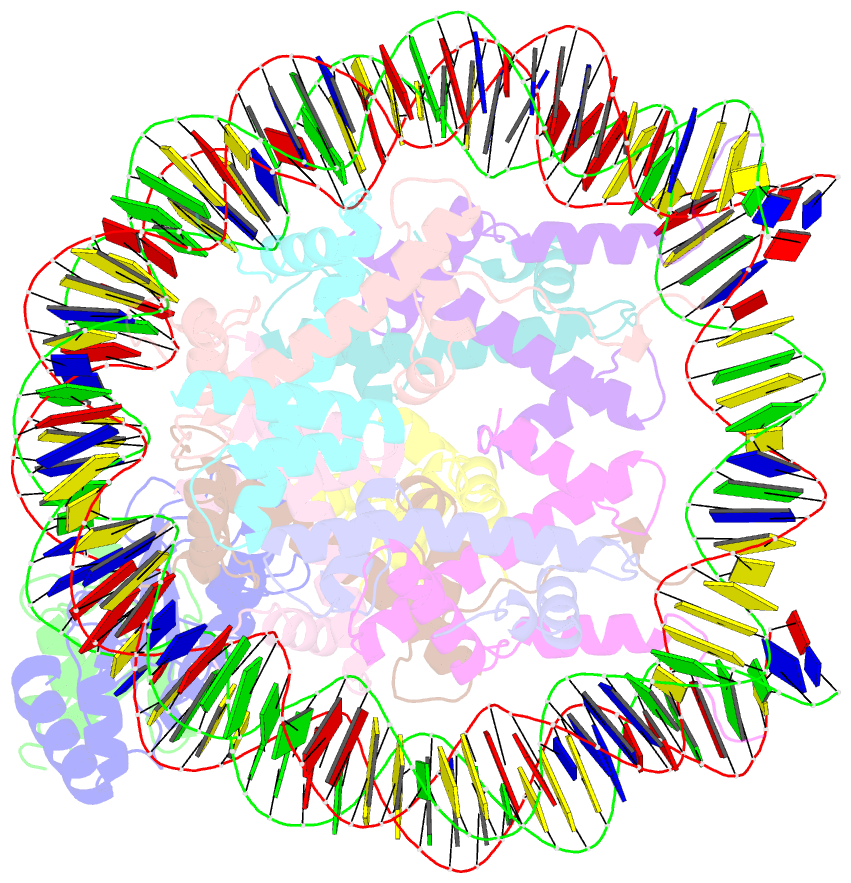
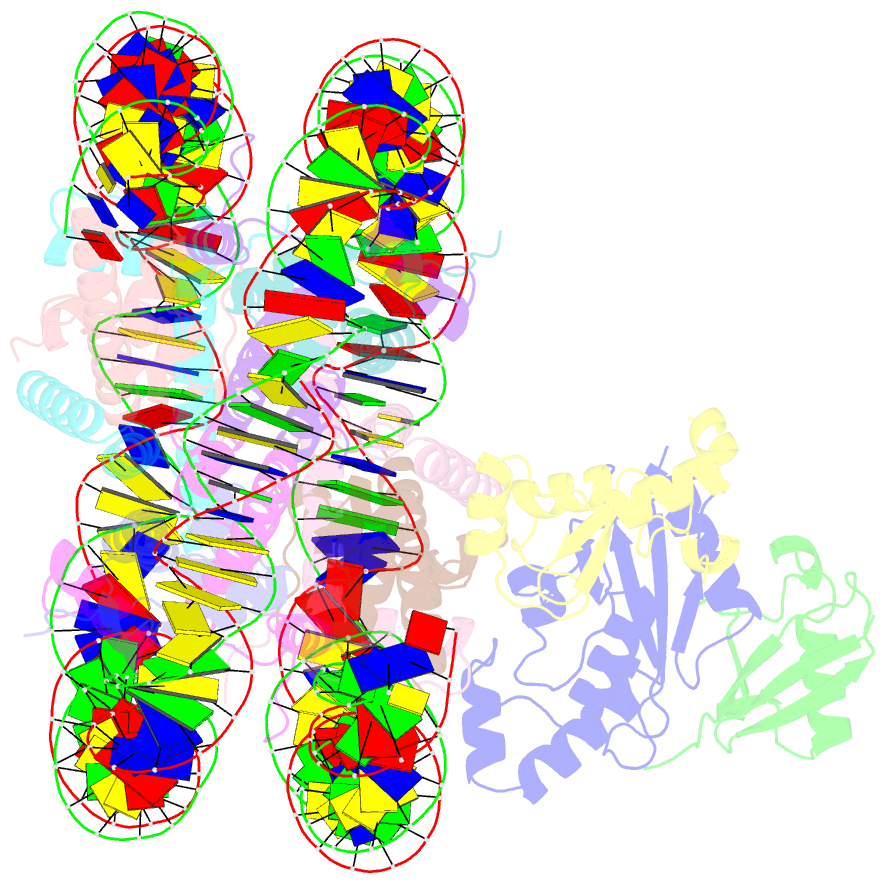
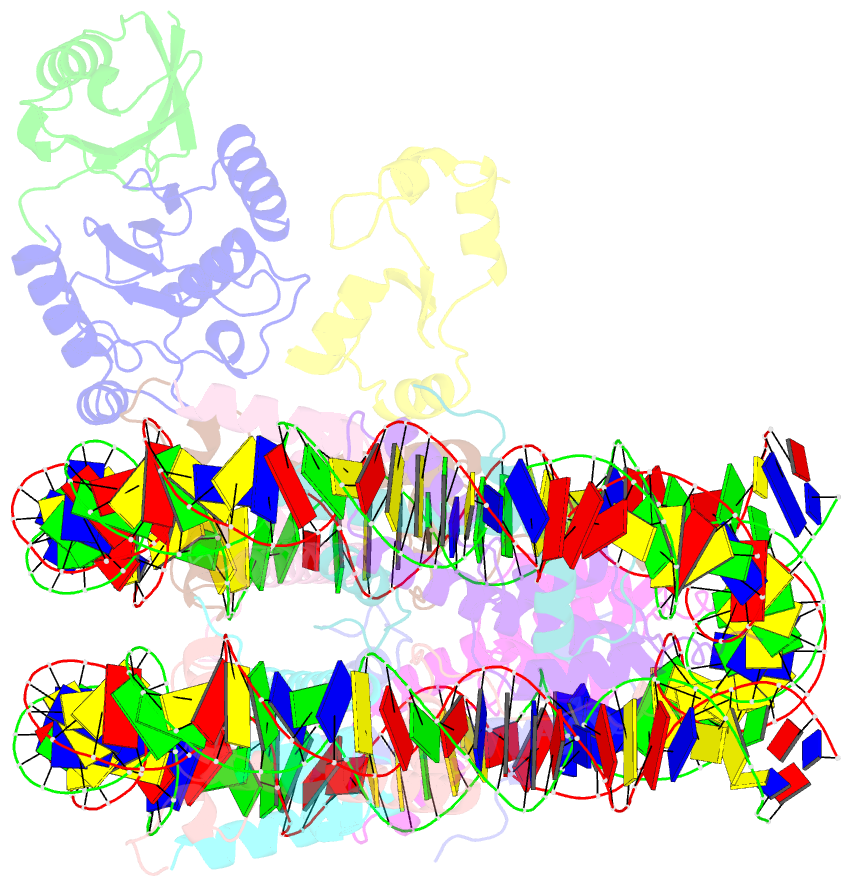
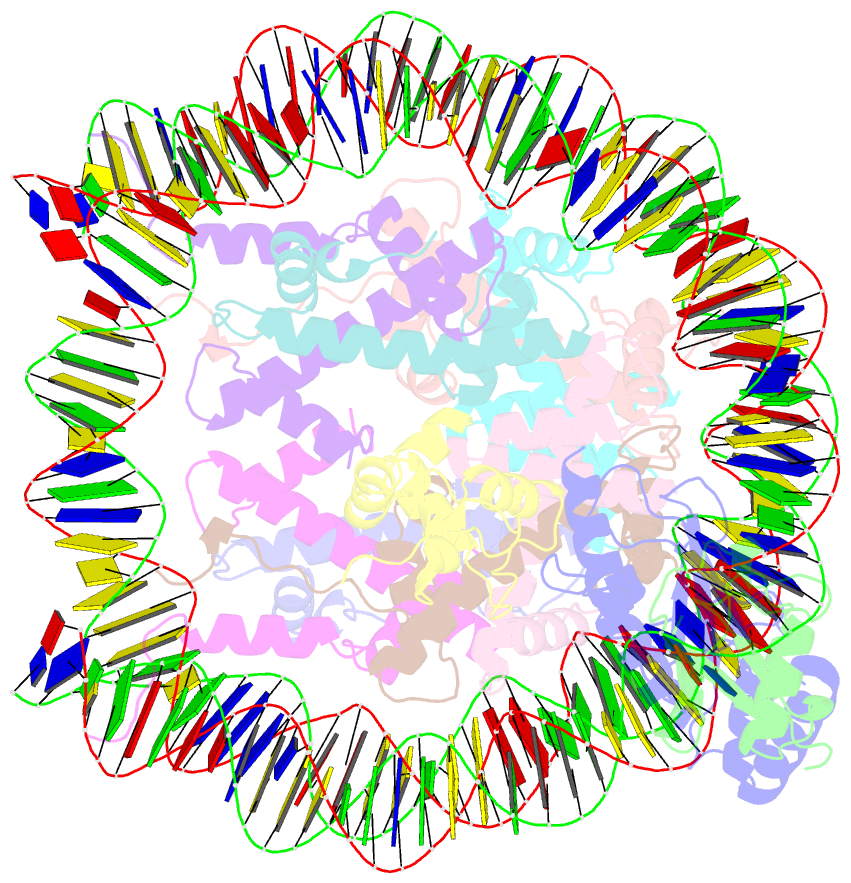
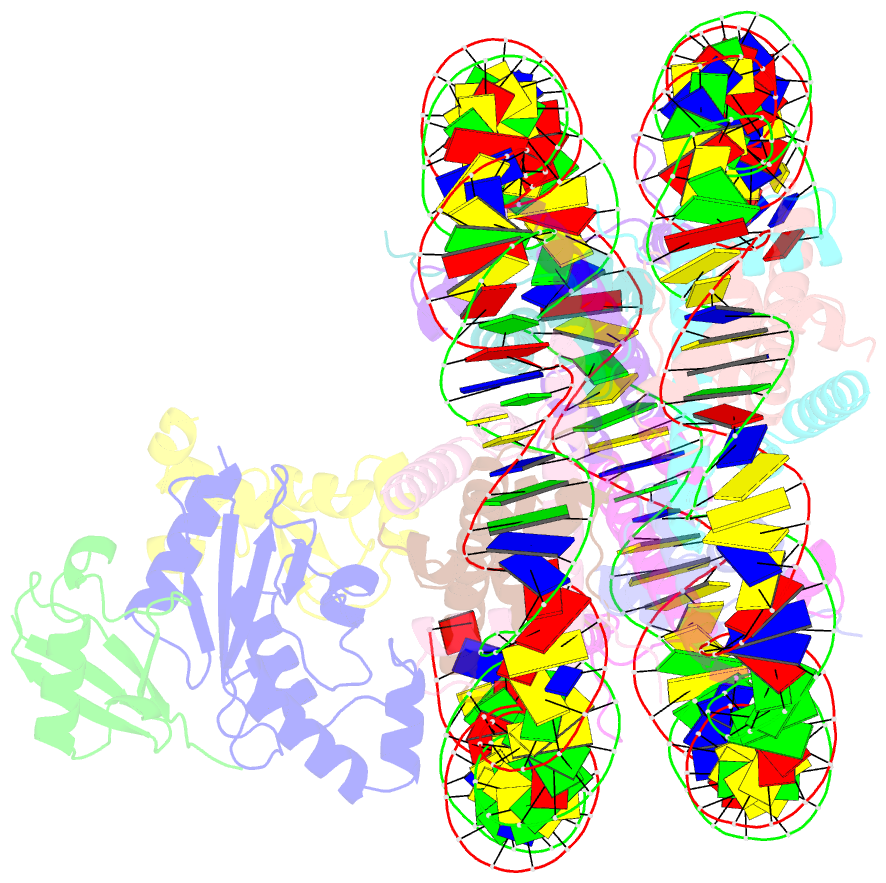
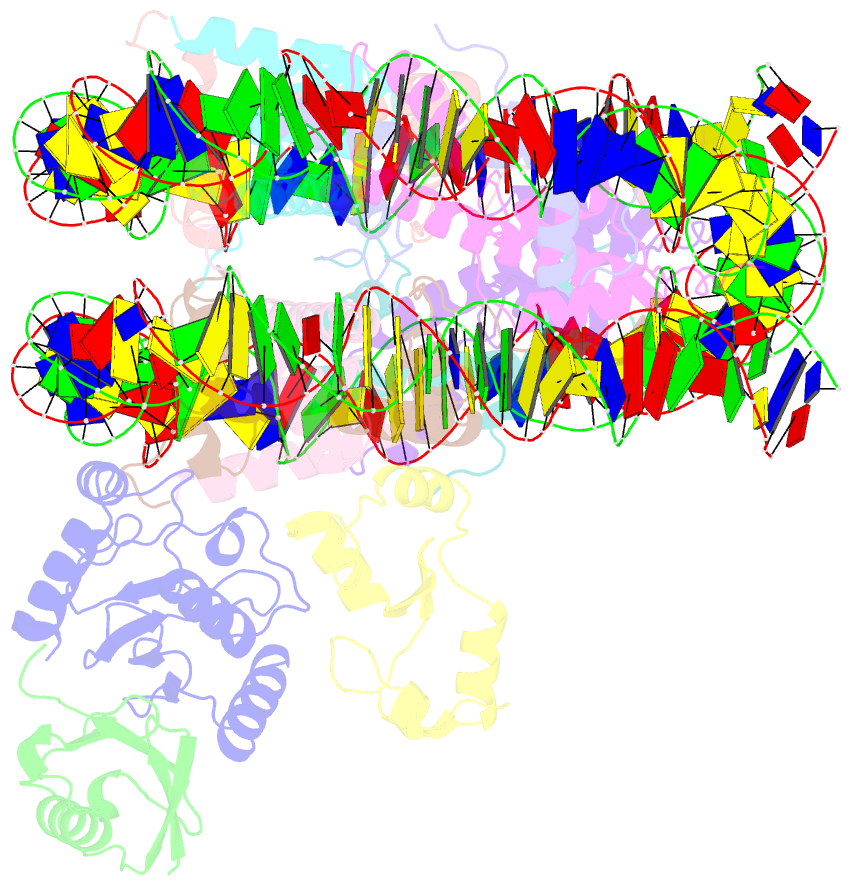
- cryo-EM structure of the human nucleosome core particle in complex with rnf168 and ubch5c with backside ubiquitin (ubch5c chemically conjugated to histone h2a) (class 2)
- Reference
- Hu Q, Zhao D, Cui G, Bhandari J, Thompson JR, Botuyan MV, Mer G (2024): "Mechanisms of RNF168 nucleosome recognition and ubiquitylation." Mol.Cell, 84, 839-853.e12. doi: 10.1016/j.molcel.2023.12.036.
- Abstract
- RNF168 plays a central role in the DNA damage response (DDR) by ubiquitylating histone H2A at K13 and K15. These modifications direct BRCA1-BARD1 and 53BP1 foci formation in chromatin, essential for cell-cycle-dependent DNA double-strand break (DSB) repair pathway selection. The mechanism by which RNF168 catalyzes the targeted accumulation of H2A ubiquitin conjugates to form repair foci around DSBs remains unclear. Here, using cryoelectron microscopy (cryo-EM), nuclear magnetic resonance (NMR) spectroscopy, and functional assays, we provide a molecular description of the reaction cycle and dynamics of RNF168 as it modifies the nucleosome and recognizes its ubiquitylation products. We demonstrate an interaction of a canonical ubiquitin-binding domain within full-length RNF168, which not only engages ubiquitin but also the nucleosome surface, clarifying how such site-specific ubiquitin recognition propels a signal amplification loop. Beyond offering mechanistic insights into a key DDR protein, our study aids in understanding site specificity in both generating and interpreting chromatin ubiquitylation.