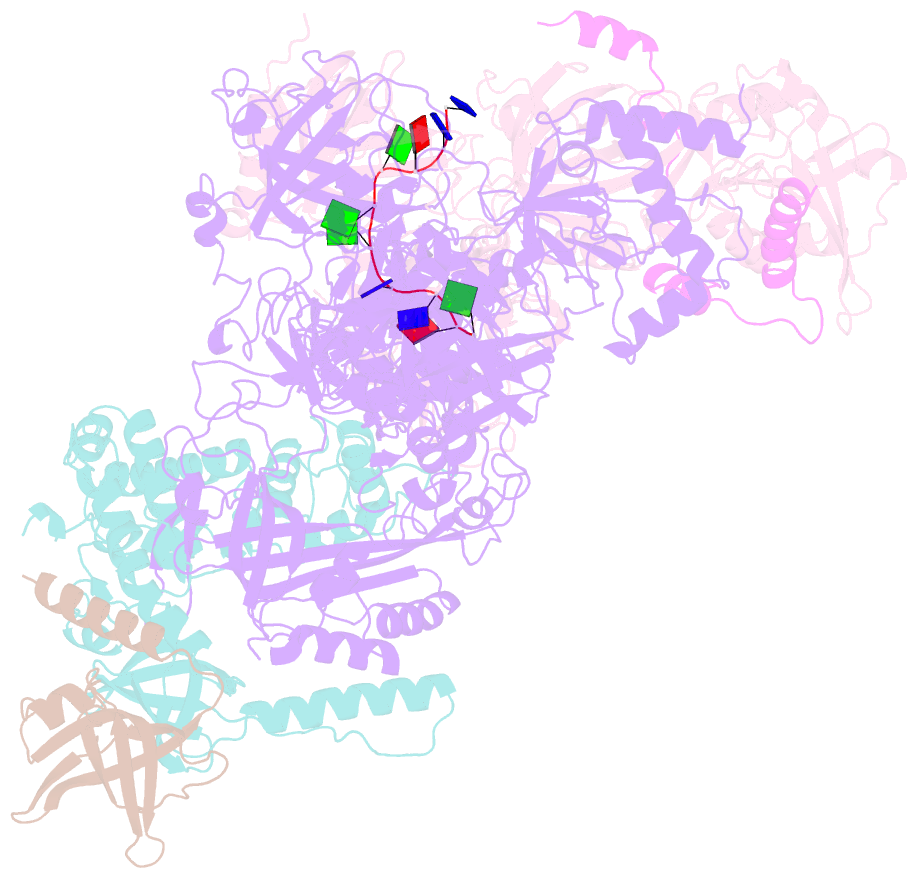
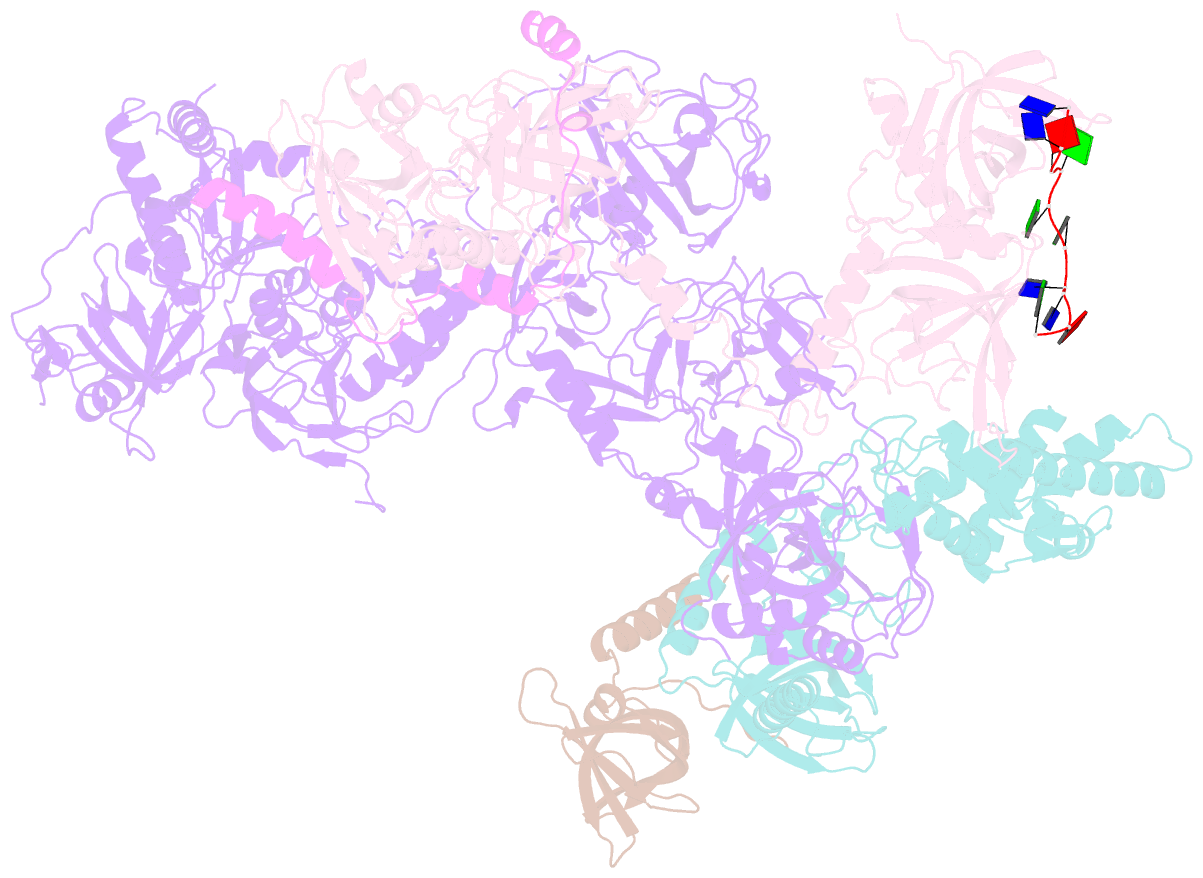
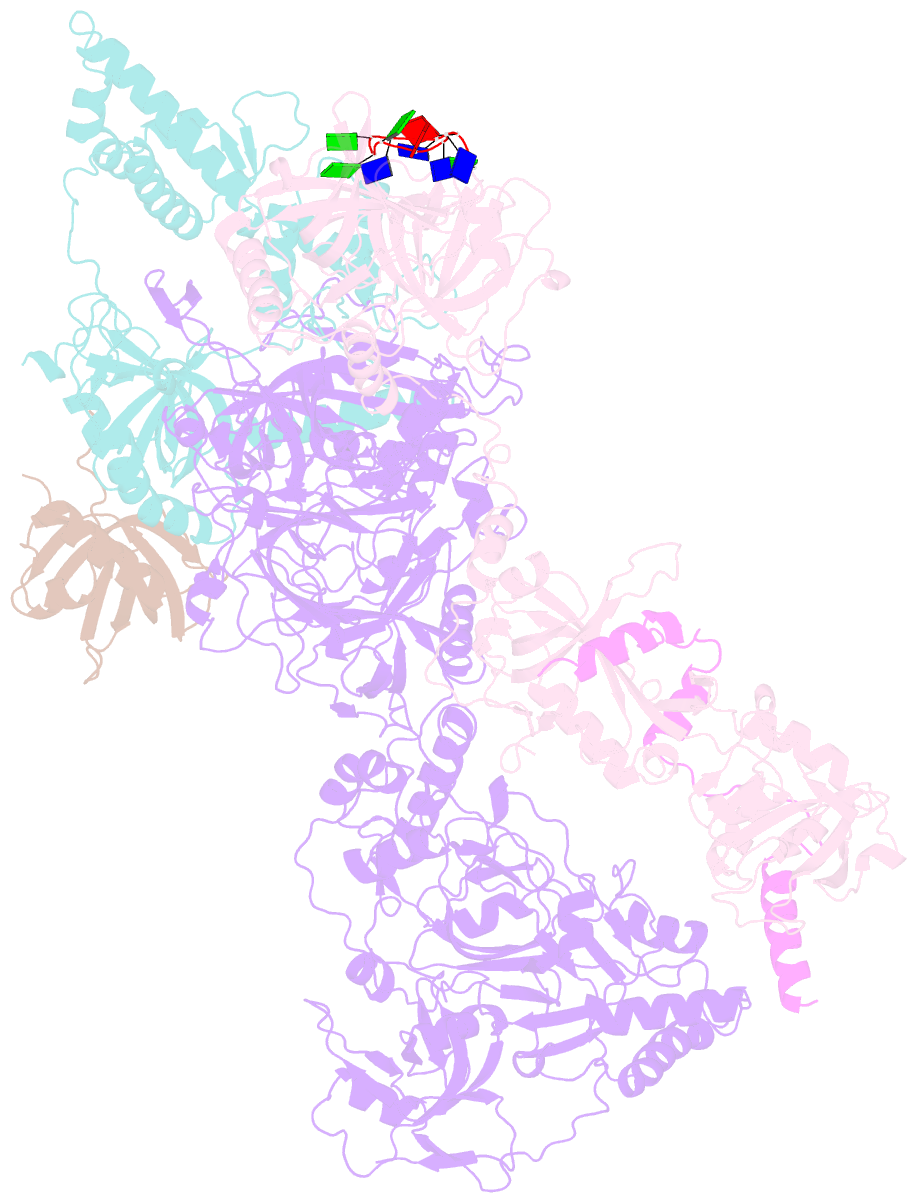
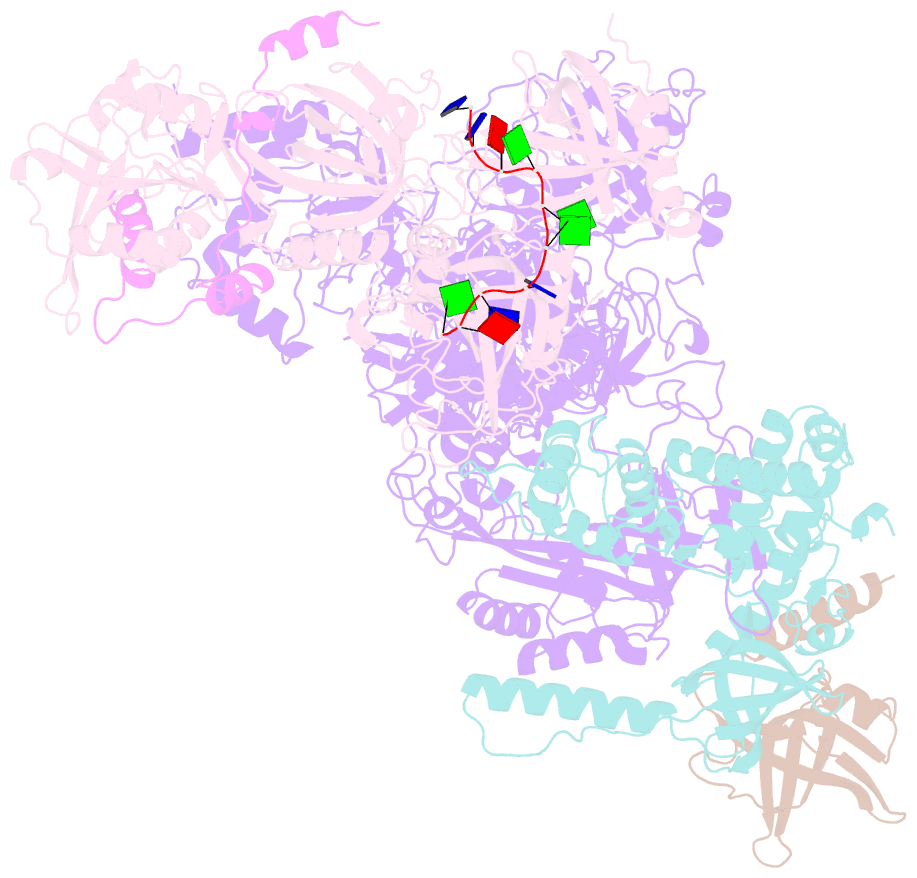
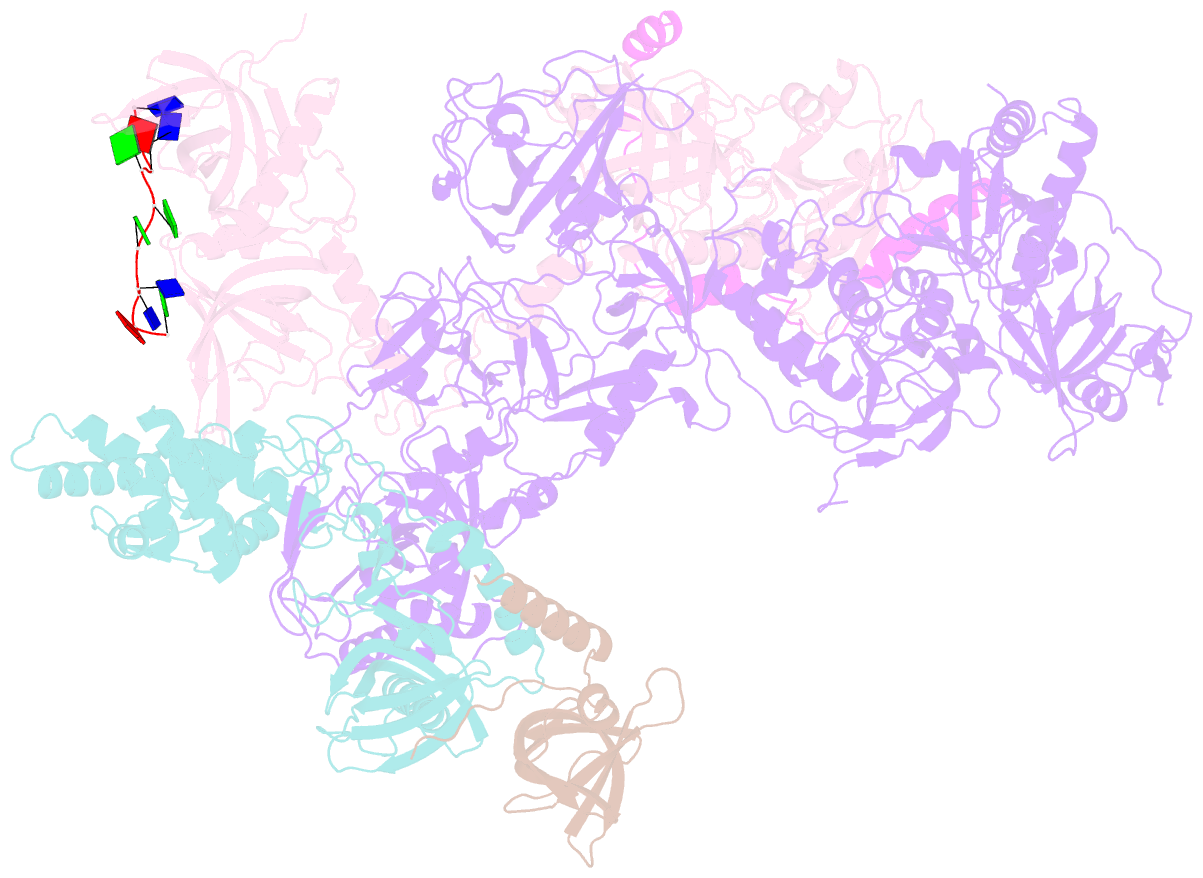
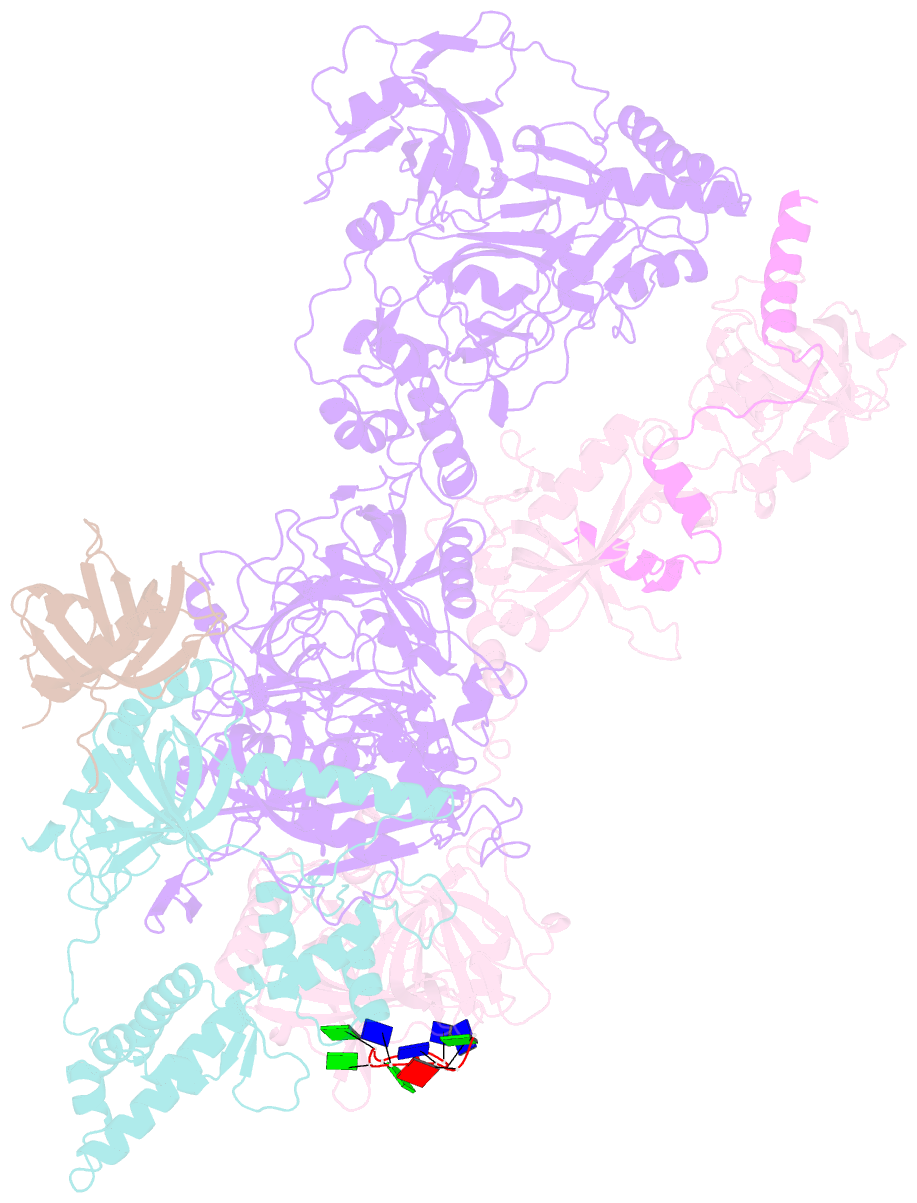
Summary information and primary citation
- PDB-id
- 8sok; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- cryo-EM (4.1 Å)
- Summary
- cryo-EM structure of human cst bound to pot1(esdl)-tpp1 in the presence of telomeric ssDNA
- Reference
- Cai SW, Takai H, Zaug AJ, Dilgen TC, Cech TR, Walz T, de Lange T (2024): "POT1 recruits and regulates CST-Pol alpha /primase at human telomeres." Cell, 187, 3638-3651.e18. doi: 10.1016/j.cell.2024.05.002.
- Abstract
- Telomere maintenance requires the extension of the G-rich telomeric repeat strand by telomerase and the fill-in synthesis of the C-rich strand by Polα/primase. At telomeres, Polα/primase is bound to Ctc1/Stn1/Ten1 (CST), a single-stranded DNA-binding complex. Like mutations in telomerase, mutations affecting CST-Polα/primase result in pathological telomere shortening and cause a telomere biology disorder, Coats plus (CP). We determined cryogenic electron microscopy structures of human CST bound to the shelterin heterodimer POT1/TPP1 that reveal how CST is recruited to telomeres by POT1. Our findings suggest that POT1 hinge phosphorylation is required for CST recruitment, and the complex is formed through conserved interactions involving several residues mutated in CP. Our structural and biochemical data suggest that phosphorylated POT1 holds CST-Polα/primase in an inactive, autoinhibited state until telomerase has extended the telomere ends. We propose that dephosphorylation of POT1 releases CST-Polα/primase into an active state that completes telomere replication through fill-in synthesis.