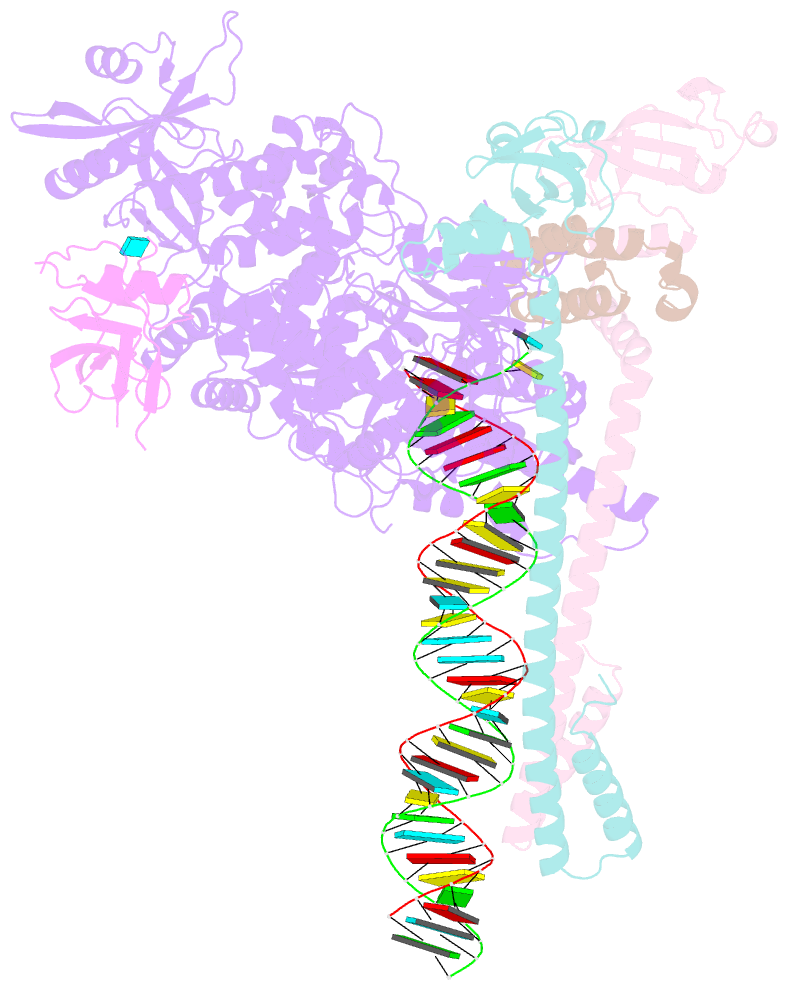
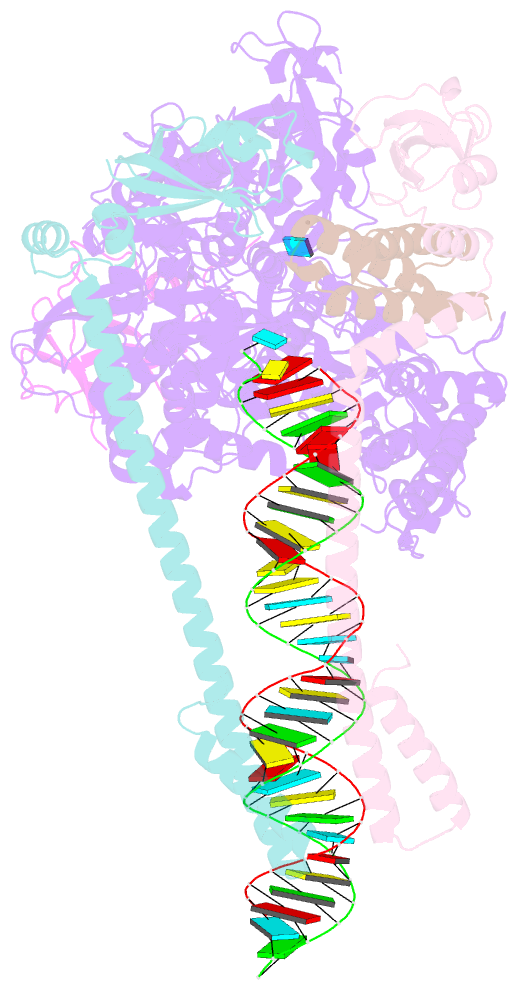
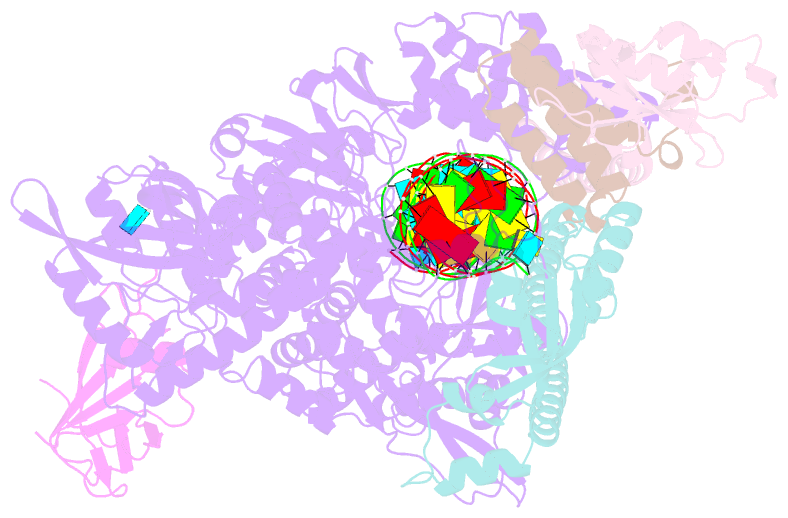
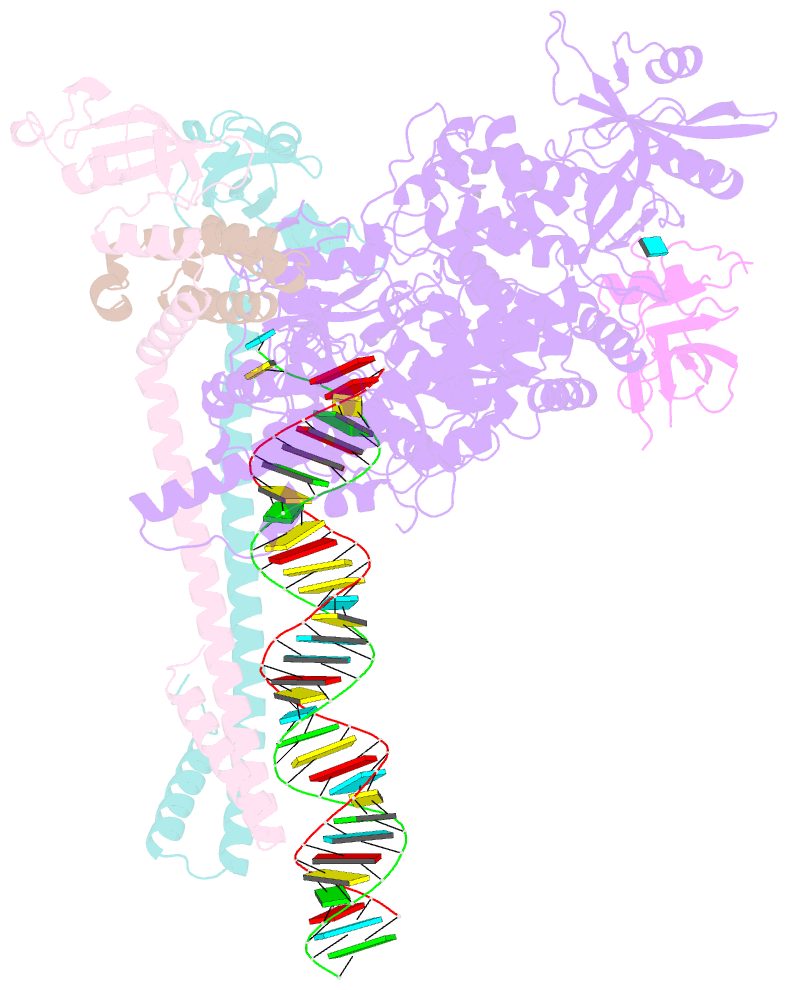
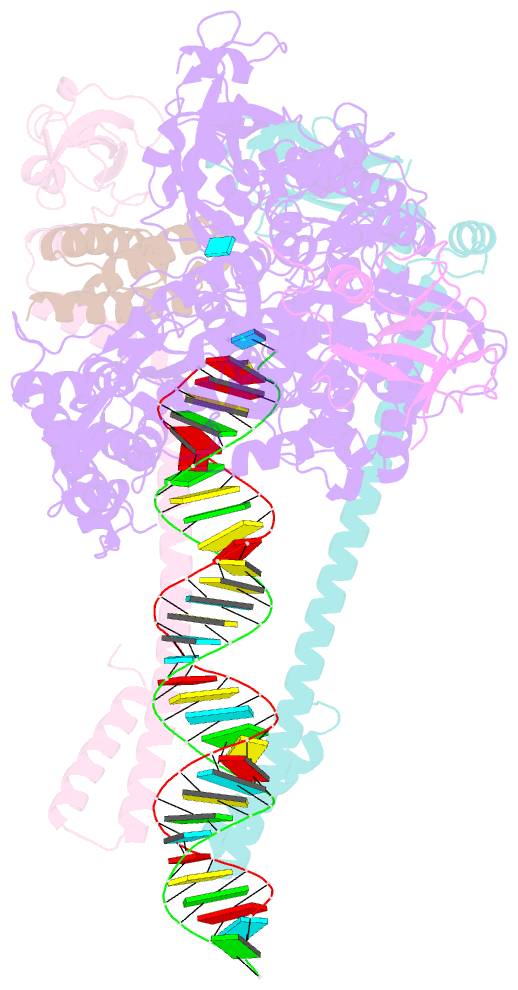
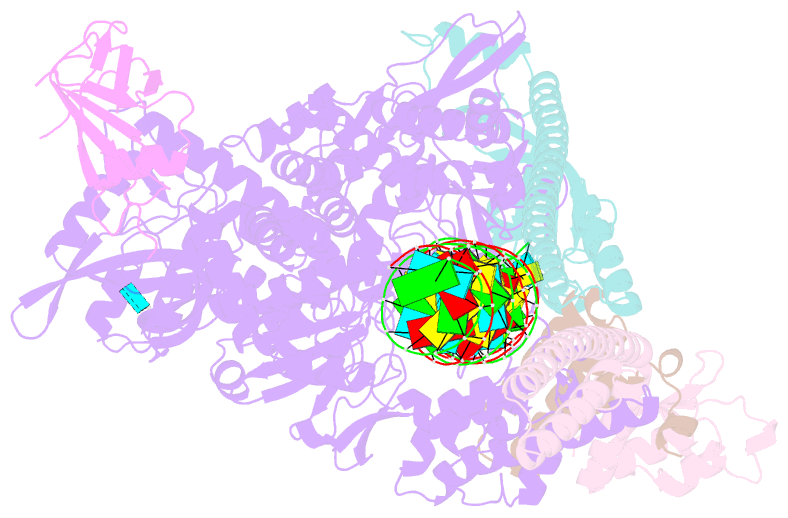
Summary information and primary citation
- PDB-id
- 8sq9; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- viral protein
- Method
- cryo-EM (2.9 Å)
- Summary
- Sars-cov-2 replication-transcription complex bound to nsp9 and umpcpp, as a pre-catalytic nmpylation intermediate
- Reference
- Small GI, Fedorova O, Olinares PDB, Chandanani J, Banerjee A, Choi YJ, Molina H, Chait BT, Darst SA, Campbell EA (2023): "Structural and functional insights into the enzymatic plasticity of the SARS-CoV-2 NiRAN domain." Mol.Cell, 83, 3921-3930.e7. doi: 10.1016/j.molcel.2023.10.001.
- Abstract
- The enzymatic activity of the SARS-CoV-2 nidovirus RdRp-associated nucleotidyltransferase (NiRAN) domain is essential for viral propagation, with three distinct activities associated with modification of the nsp9 N terminus, NMPylation, RNAylation, and deRNAylation/capping via a GDP-polyribonucleotidyltransferase reaction. The latter two activities comprise an unconventional mechanism for initiating viral RNA 5' cap formation, while the role of NMPylation is unclear. The structural mechanisms for these diverse enzymatic activities have not been properly delineated. Here, we determine high-resolution cryoelectron microscopy (cryo-EM) structures of catalytic intermediates for the NMPylation and deRNAylation/capping reactions, revealing diverse nucleotide binding poses and divalent metal ion coordination sites to promote its repertoire of activities. The deRNAylation/capping structure explains why GDP is a preferred substrate for the capping reaction over GTP. Altogether, these findings enhance our understanding of the promiscuous coronaviral NiRAN domain, a therapeutic target, and provide an accurate structural platform for drug development.