Summary information and primary citation
- PDB-id
- 8t3t; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-transferase-DNA
- Method
- cryo-EM (3.21 Å)
- Summary
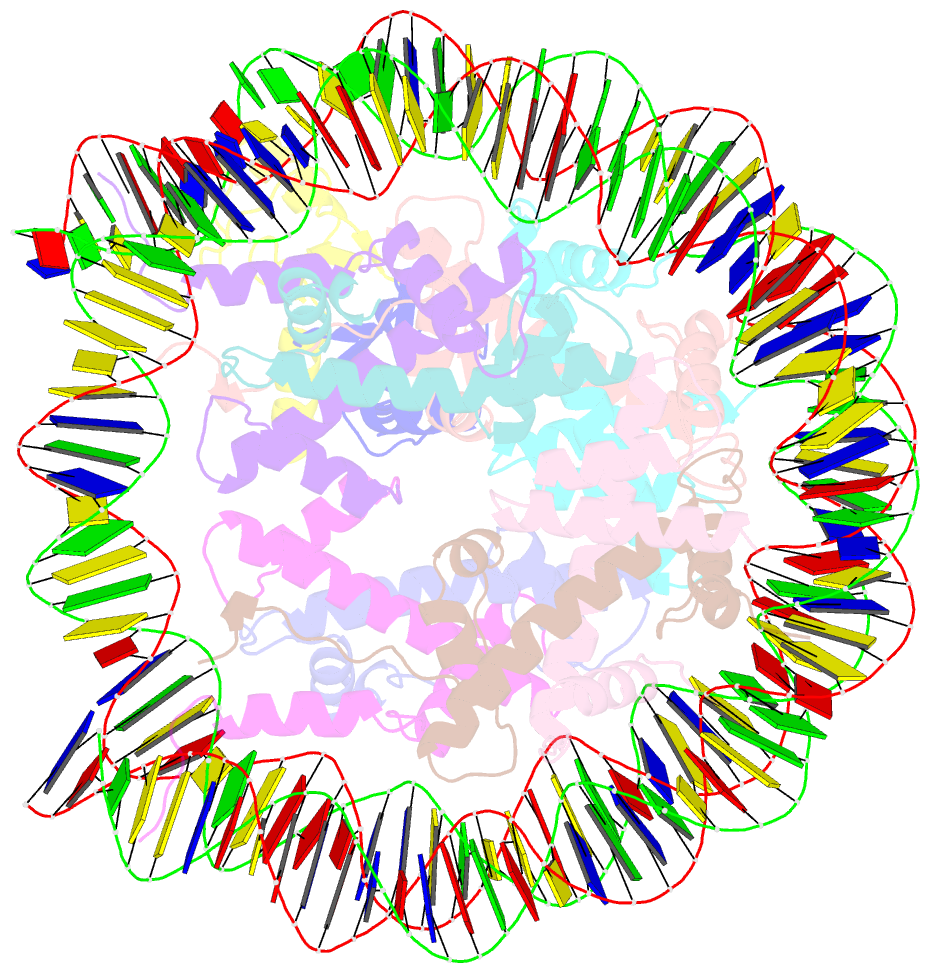
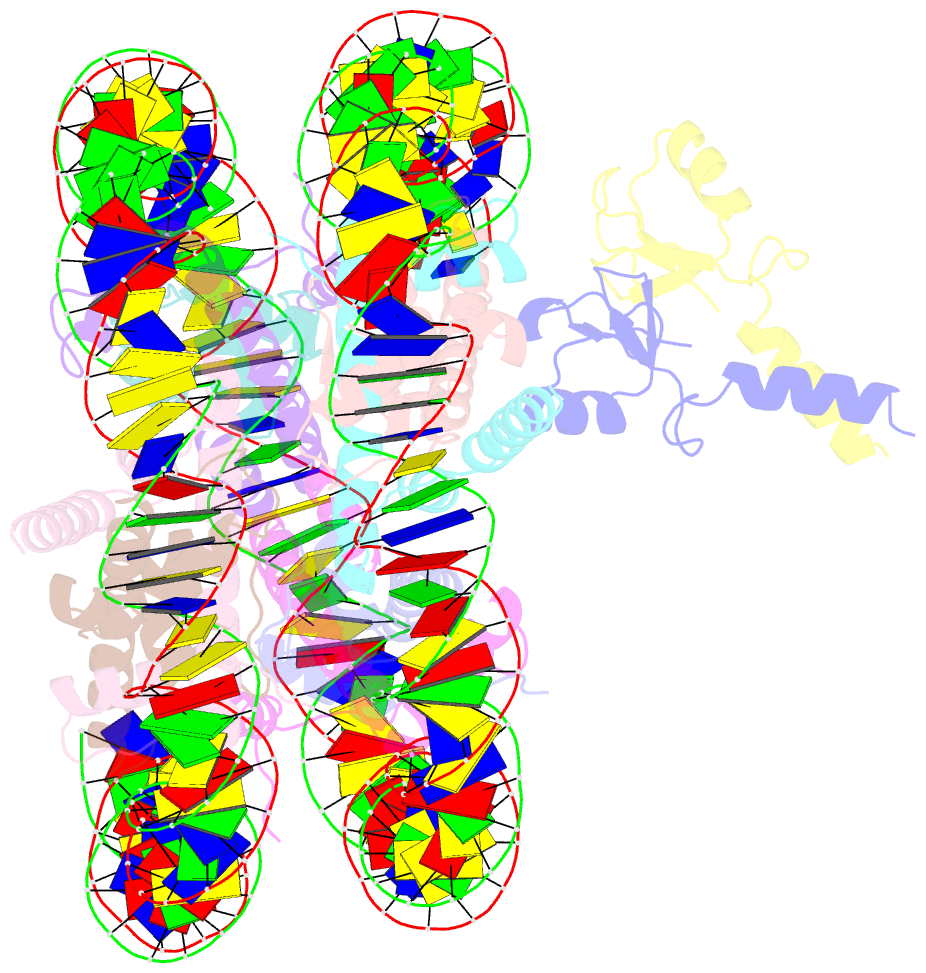
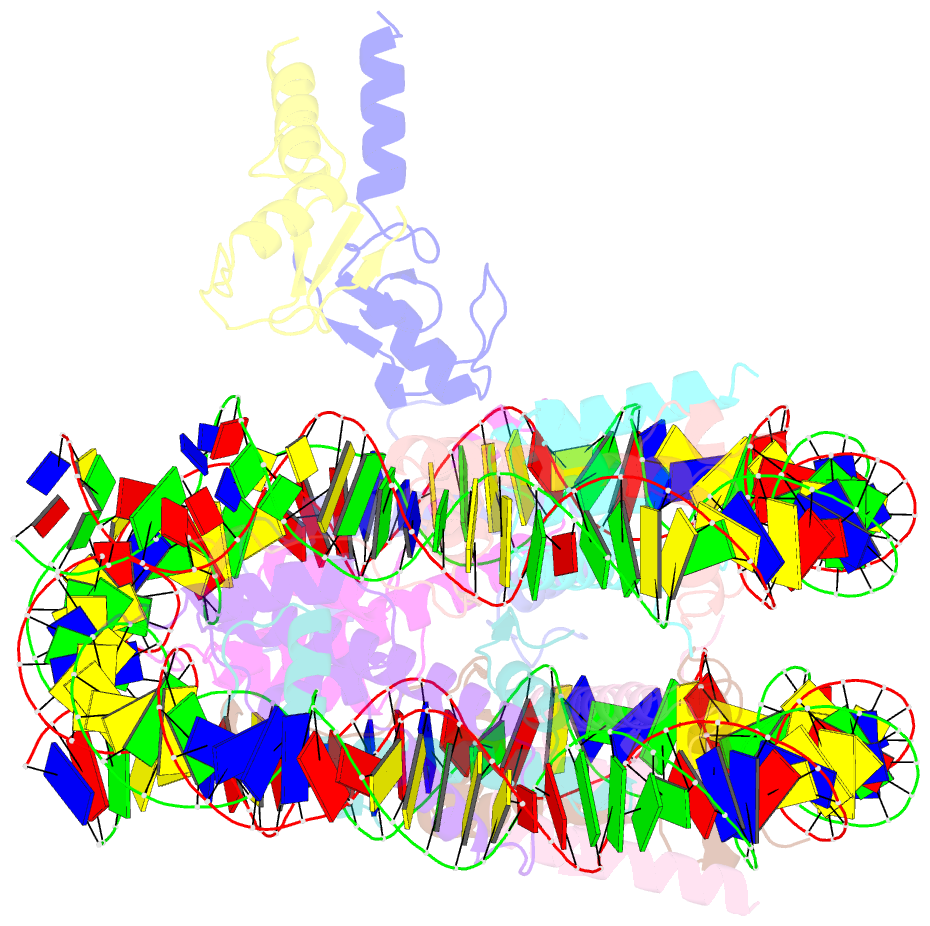
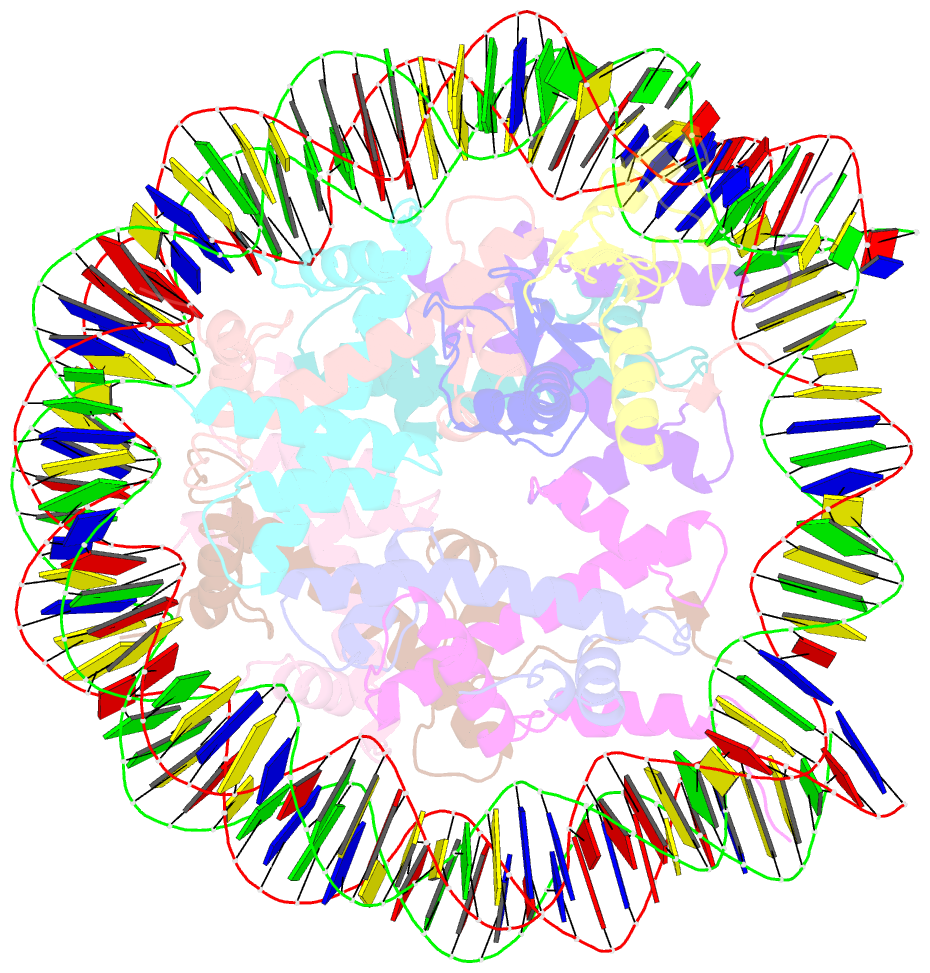
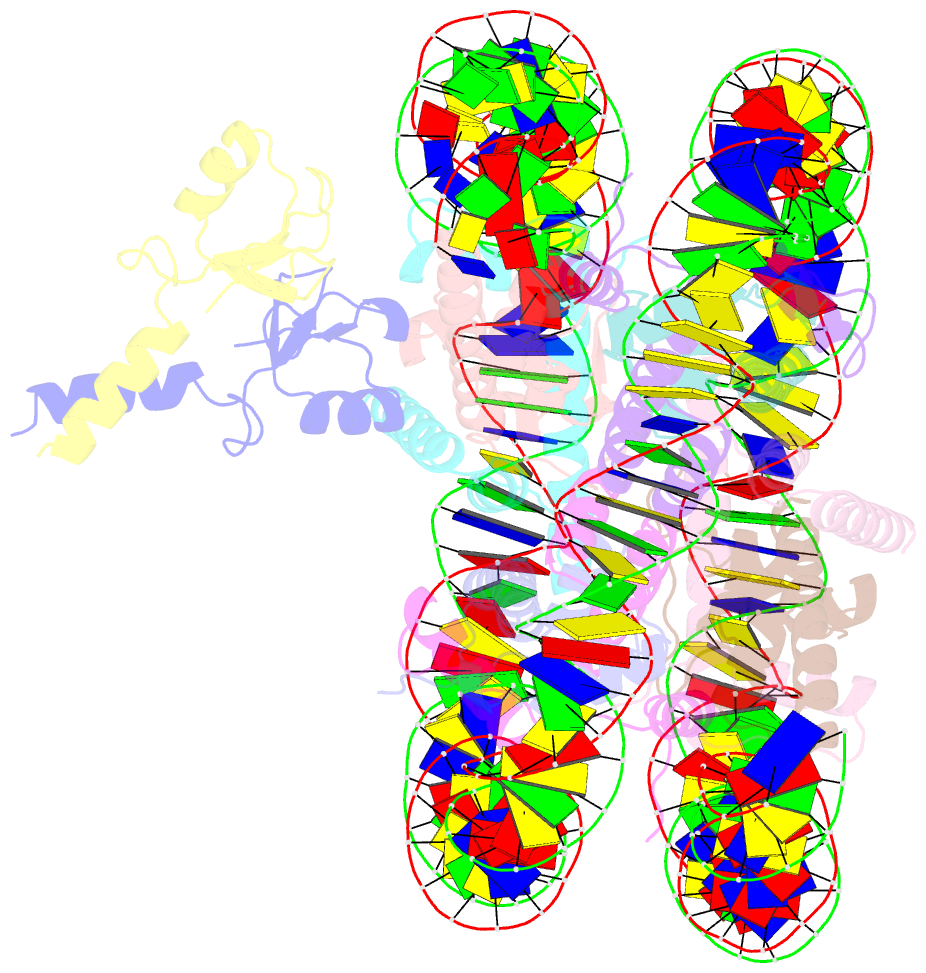
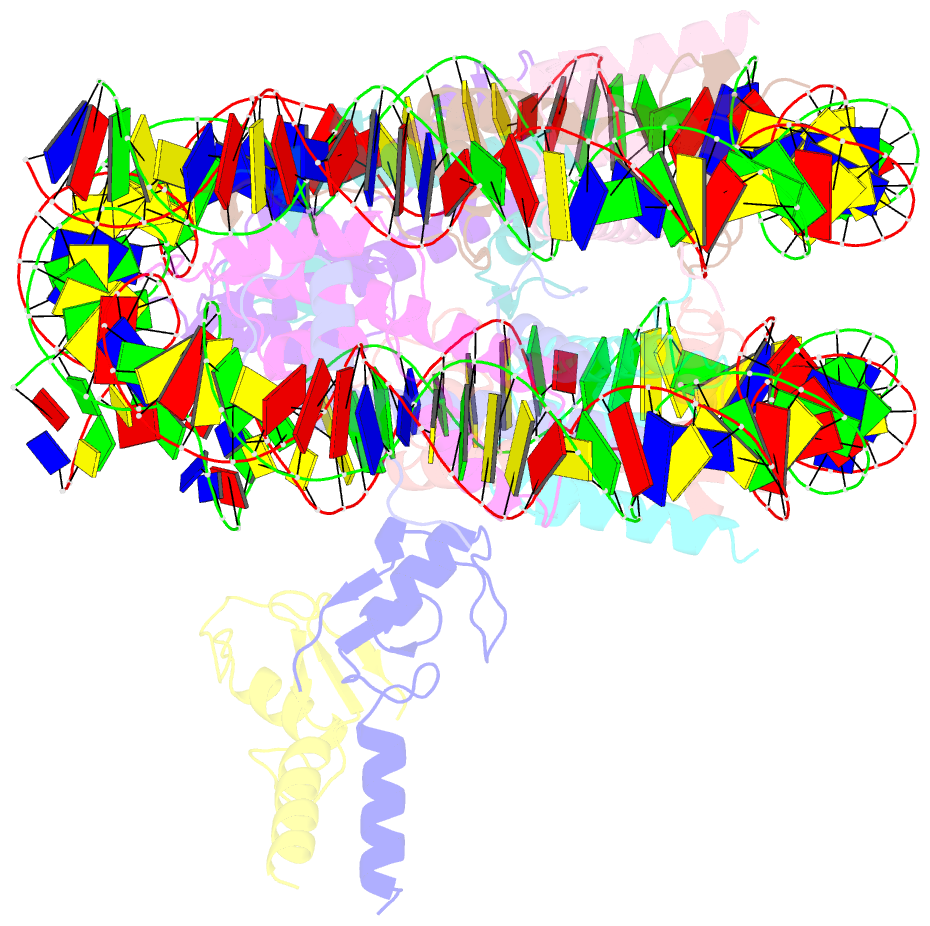
- Structure of bre1-nucleosome complex - state3
- Reference
- Zhao F, Hicks CW, Wolberger C (2023): "Mechanism of histone H2B monoubiquitination by Bre1." Nat.Struct.Mol.Biol., 30, 1623-1627. doi: 10.1038/s41594-023-01137-x.
- Abstract
- Monoubiquitination of histone H2B-K120/123 plays several roles in regulating transcription, DNA replication and the DNA damage response. The structure of a nucleosome in complex with the dimeric RING E3 ligase Bre1 reveals that one RING domain binds to the nucleosome acidic patch, where it can position the E2 ubiquitin conjugating enzyme Rad6, while the other RING domain contacts the DNA. Comparisons with H2A-specific E3 ligases suggest a general mechanism of tuning histone specificity via the non-E2-binding RING domain.