Summary information and primary citation
- PDB-id
- 8t4s; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome-viral protein
- Method
- cryo-EM (2.6 Å)
- Summary
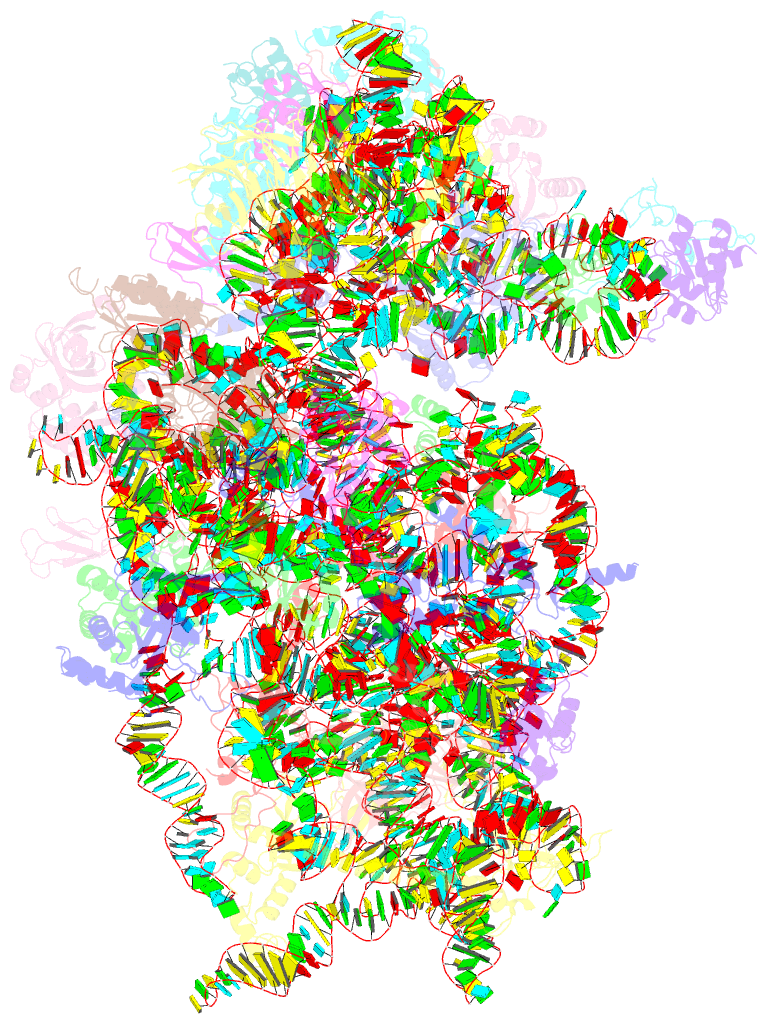
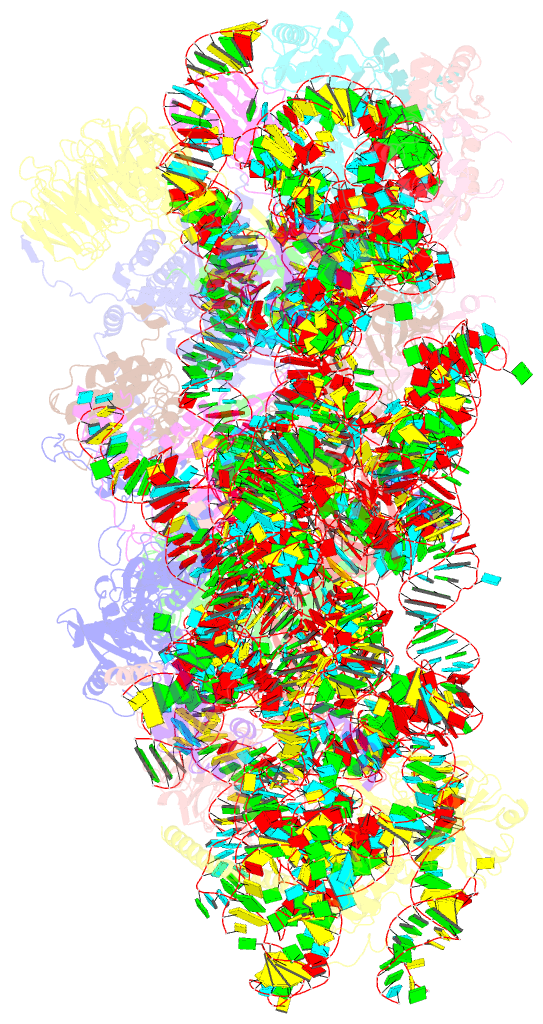
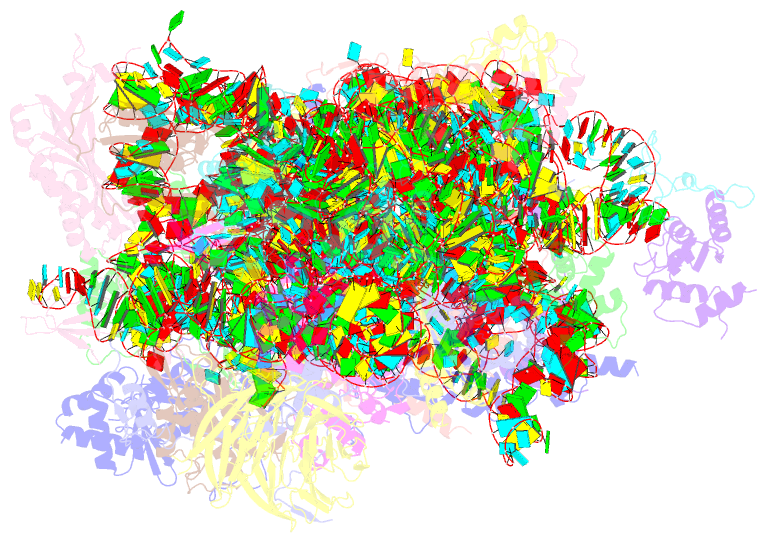
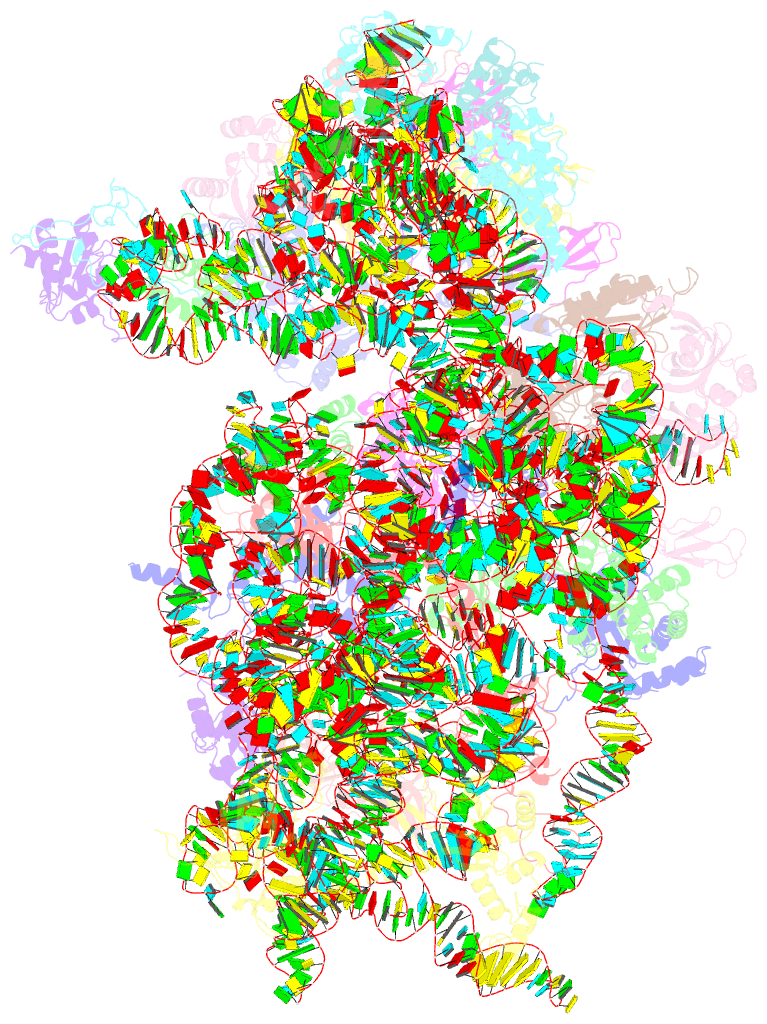
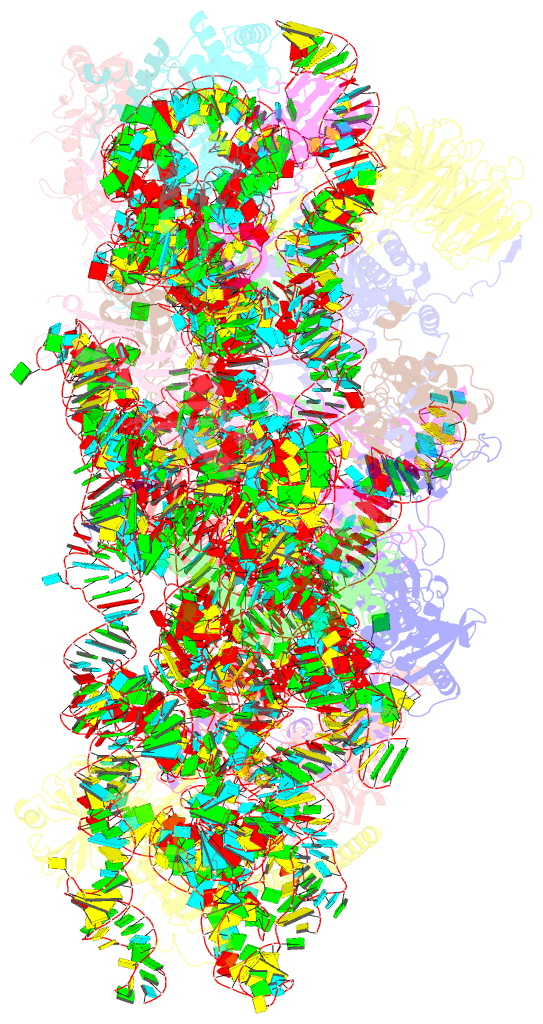
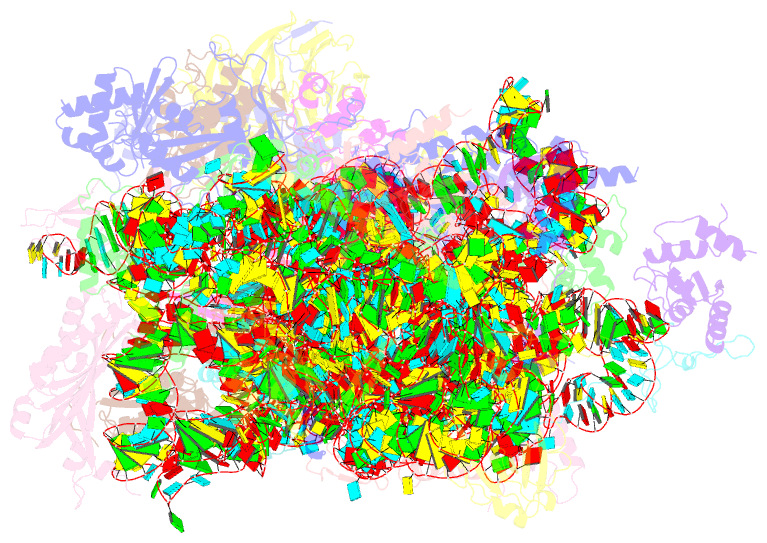
- Mers-cov nsp1 protein bound to the human 40s ribosomal subunit
- Reference
- Devarkar SC, Vetick M, Balaji S, Lomakin IB, Yang L, Jin D, Gilbert WV, Chen S, Xiong Y (2023): "Structural basis for translation inhibition by MERS-CoV Nsp1 reveals a conserved mechanism for betacoronaviruses." Cell Rep, 42, 113156. doi: 10.1016/j.celrep.2023.113156.
- Abstract
- All betacoronaviruses (β-CoVs) encode non-structural protein 1 (Nsp1), an essential pathogenicity factor that potently restricts host gene expression. Among the β-CoV family, MERS-CoV is the most distantly related member to SARS-CoV-2, and the mechanism for host translation inhibition by MERS-CoV Nsp1 remains controversial. Herein, we show that MERS-CoV Nsp1 directly interacts with the 40S ribosomal subunit. Using cryogenic electron microscopy (cryo-EM), we report a 2.6-Å structure of the MERS-CoV Nsp1 bound to the human 40S ribosomal subunit. The extensive interactions between C-terminal domain of MERS-CoV Nsp1 and the mRNA entry channel of the 40S ribosomal subunit are critical for its translation inhibition function. This mechanism of MERS-CoV Nsp1 is strikingly similar to SARS-CoV and SARS-CoV-2 Nsp1, despite modest sequence conservation. Our results reveal that the mechanism of host translation inhibition is conserved across β-CoVs and highlight a potential therapeutic target for the development of antivirals that broadly restrict β-CoVs.