Summary information and primary citation
- PDB-id
- 8u3b; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- cryo-EM (3.23 Å)
- Summary
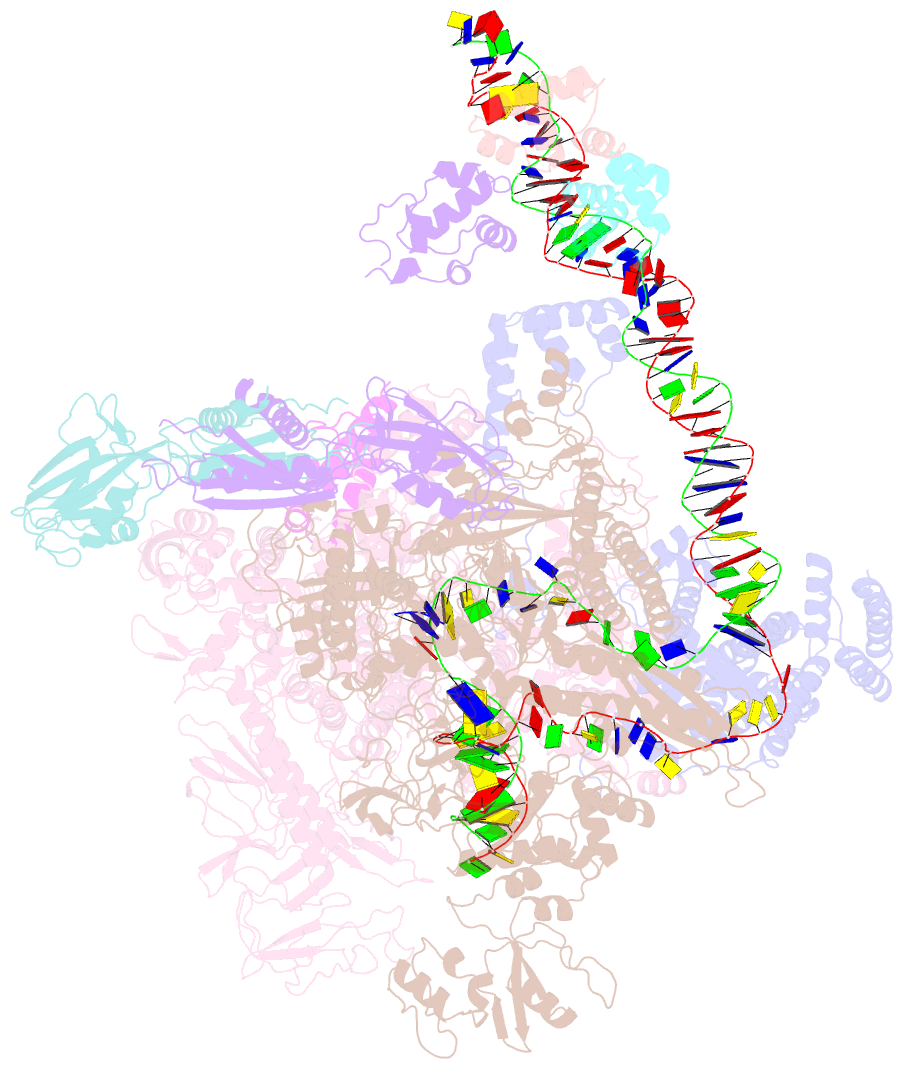
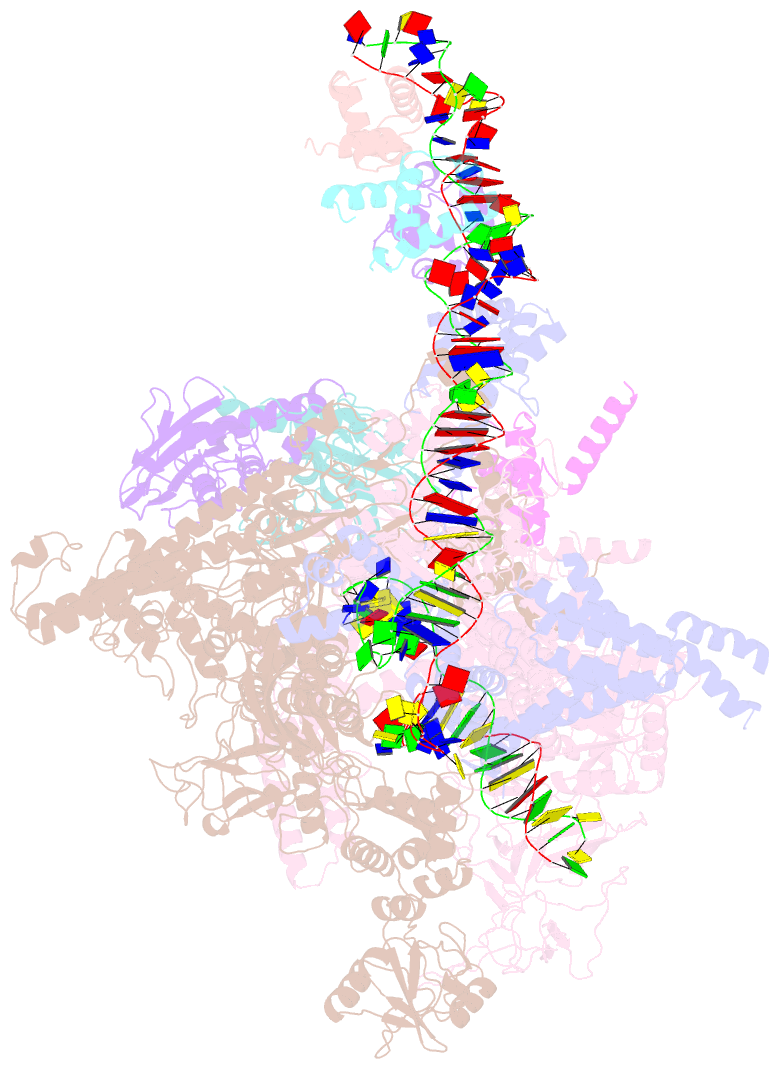
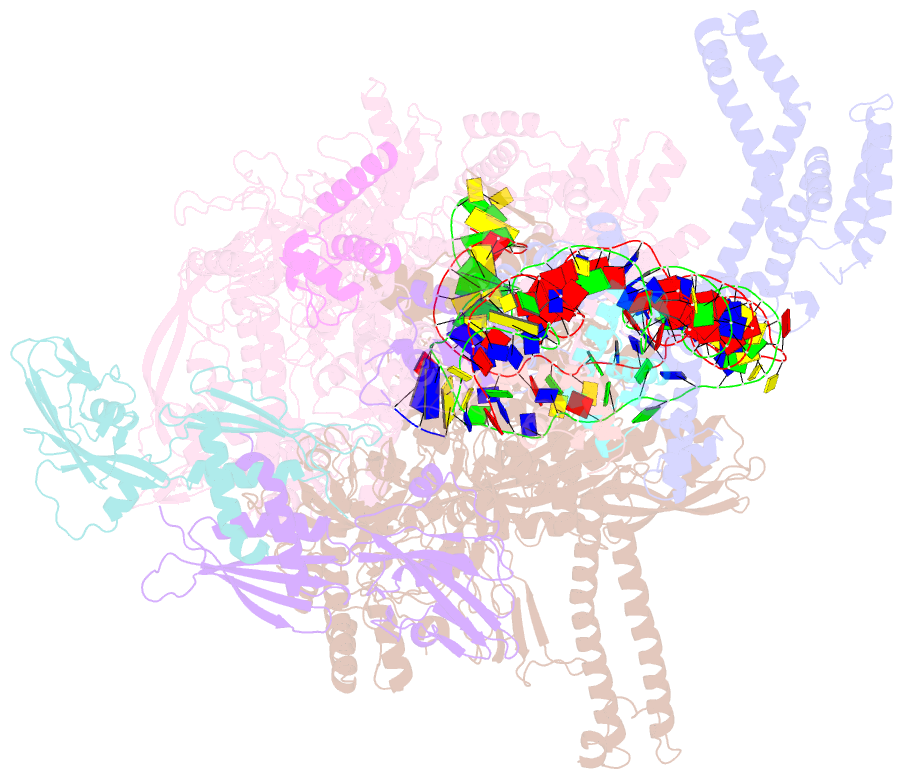
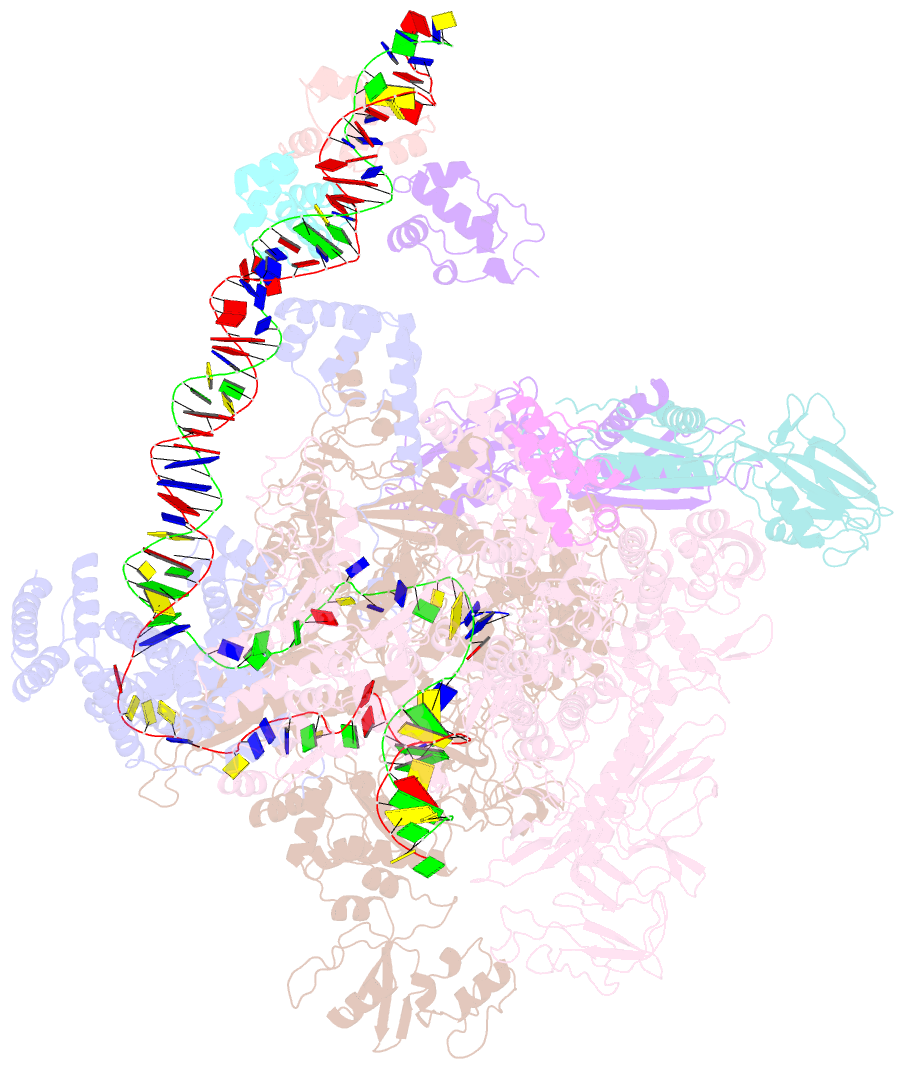
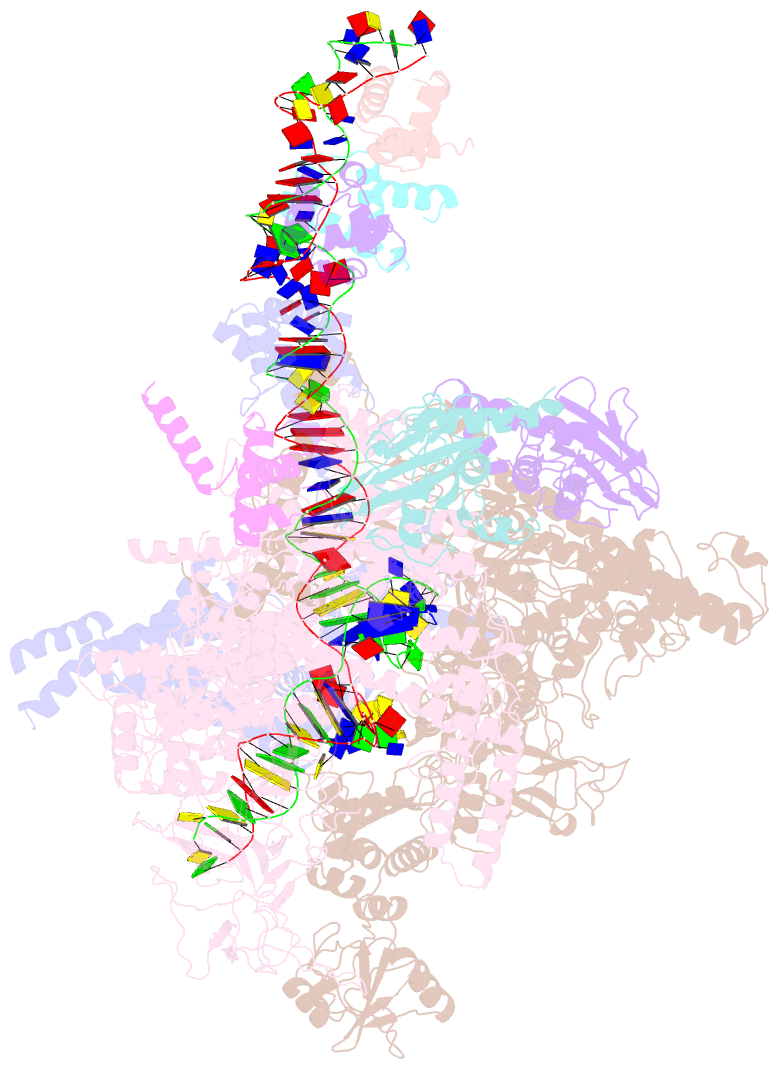
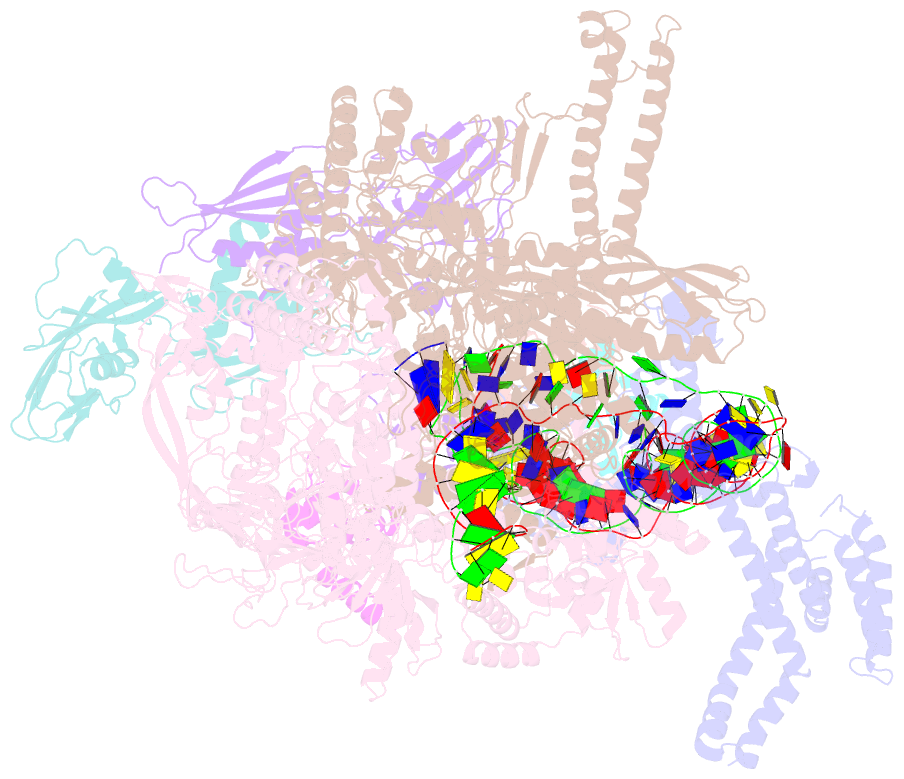
- cryo-EM structure of e. coli narl-transcription activation complex at 3.2a
- Reference
- Kompaniiets D, He L, Wang D, Zhou W, Yang Y, Hu Y, Liu B (2024): "Structural basis for transcription activation by the nitrate-responsive regulator NarL." Nucleic Acids Res., 52, 1471-1482. doi: 10.1093/nar/gkad1231.
- Abstract
- Transcription activation is a crucial step of regulation during transcription initiation and a classic check point in response to different stimuli and stress factors. The Escherichia coli NarL is a nitrate-responsive global transcription factor that controls the expression of nearly 100 genes. However, the molecular mechanism of NarL-mediated transcription activation is not well defined. Here we present a cryo-EM structure of NarL-dependent transcription activation complex (TAC) assembled on the yeaR promoter at 3.2 Å resolution. Our structure shows that the NarL dimer binds at the -43.5 site of the promoter DNA with its C-terminal domain (CTD) not only binding to the DNA but also making interactions with RNA polymerase subunit alpha CTD (αCTD). The key role of these NarL-mediated interactions in transcription activation was further confirmed by in vivo and in vitro transcription assays. Additionally, the NarL dimer binds DNA in a different plane from that observed in the structure of class II TACs. Unlike the canonical class II activation mechanism, NarL does not interact with σ4, while RNAP αCTD is bound to DNA on the opposite side of NarL. Our findings provide a structural basis for detailed mechanistic understanding of NarL-dependent transcription activation on yeaR promoter and reveal a potentially novel mechanism of transcription activation.