Summary information and primary citation
- PDB-id
- 8uua; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- cryo-EM (2.7 Å)
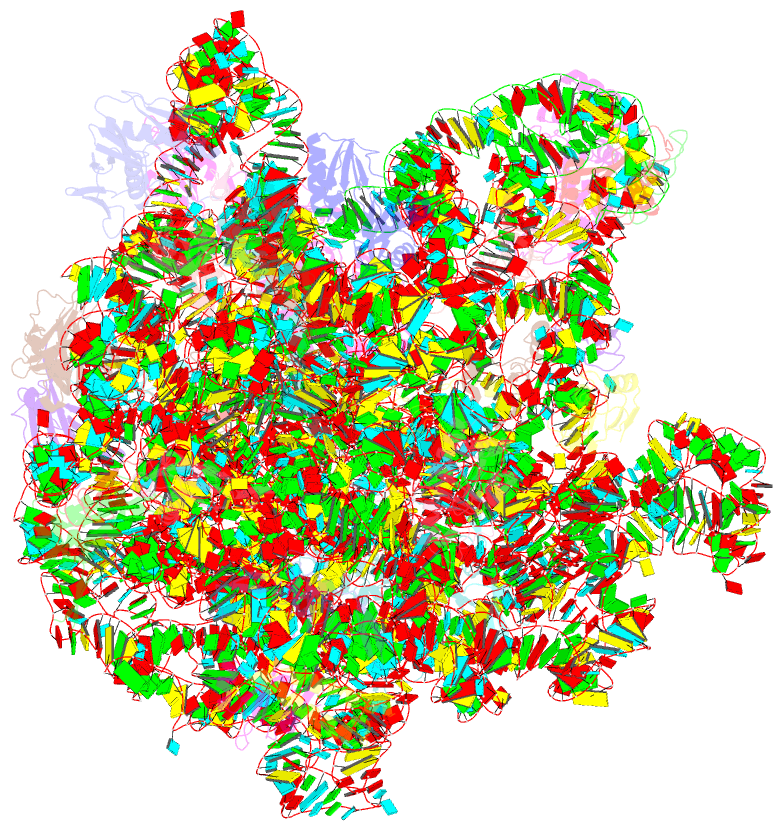
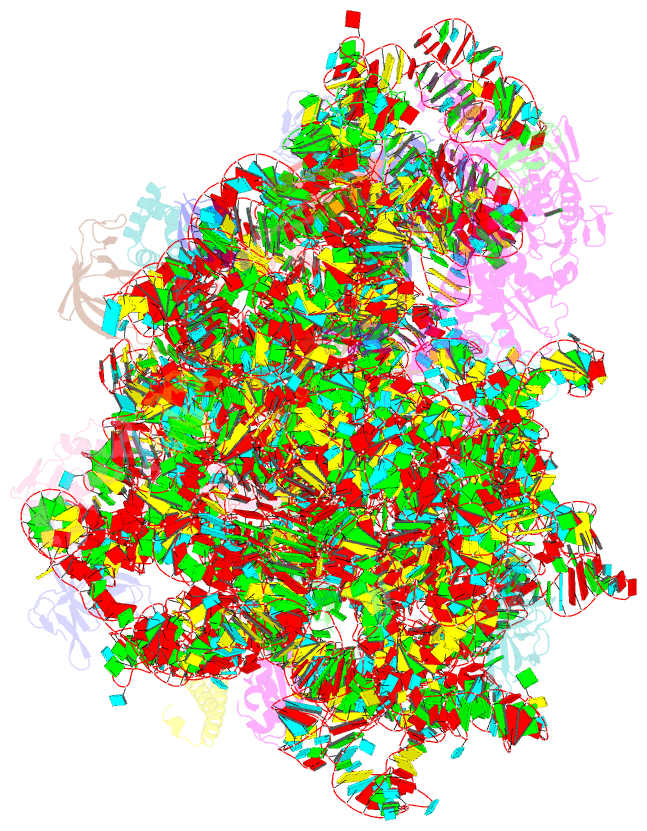
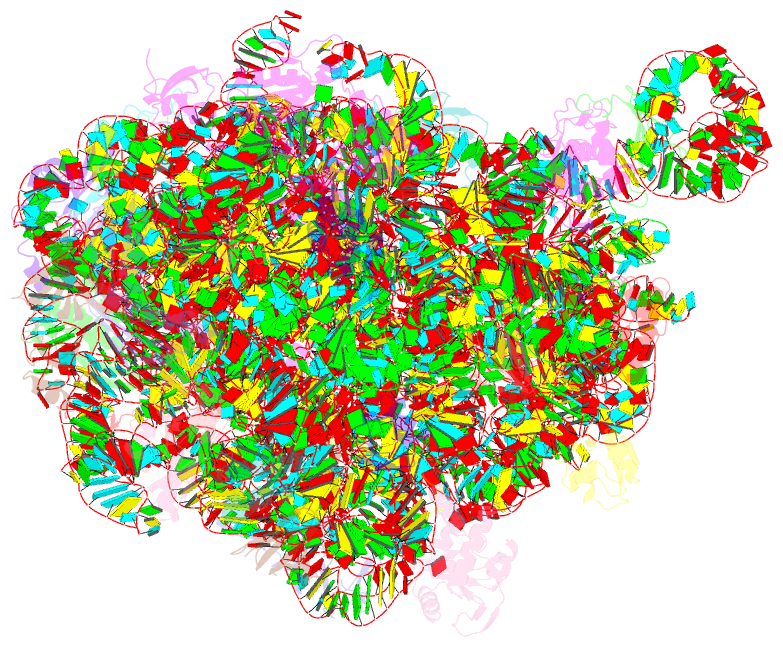
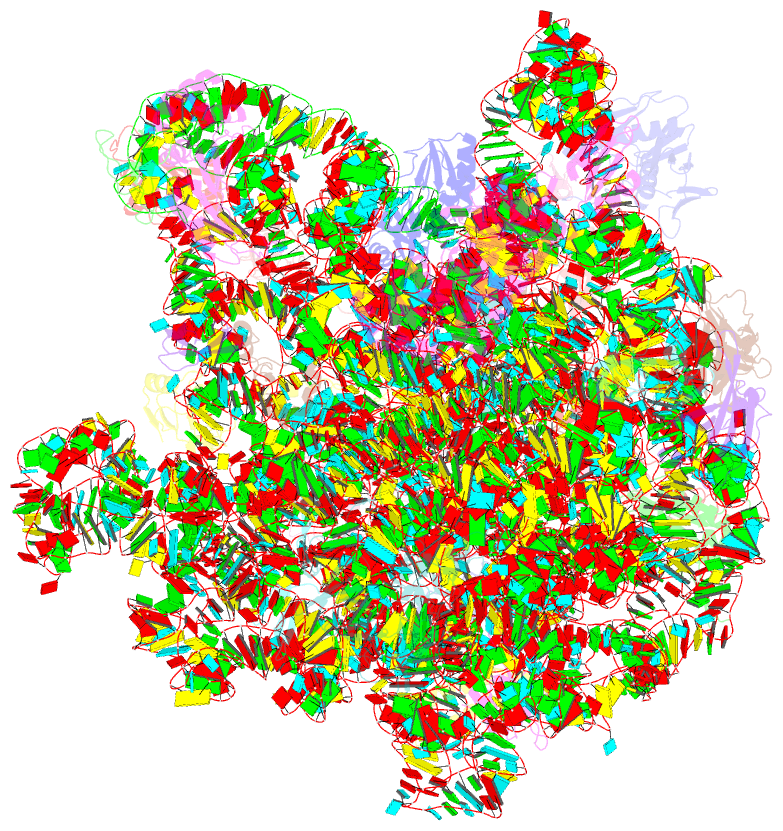
- Summary
- cryo-EM structure of the listeria innocua 50s ribosomal subunit in complex with hflxr (structure iii)
- Reference
- Seely SM, Basu RS, Gagnon MG (2024): "Mechanistic insights into the alternative ribosome recycling by HflXr." Nucleic Acids Res., 52, 4053-4066. doi: 10.1093/nar/gkae128.
- Abstract
- During stress conditions such as heat shock and antibiotic exposure, ribosomes stall on messenger RNAs, leading to inhibition of protein synthesis. To remobilize ribosomes, bacteria use rescue factors such as HflXr, a homolog of the conserved housekeeping GTPase HflX that catalyzes the dissociation of translationally inactive ribosomes into individual subunits. Here we use time-resolved cryo-electron microscopy to elucidate the mechanism of ribosome recycling by Listeria monocytogenes HflXr. Within the 70S ribosome, HflXr displaces helix H69 of the 50S subunit and induces long-range movements of the platform domain of the 30S subunit, disrupting inter-subunit bridges B2b, B2c, B4, B7a and B7b. Our findings unveil a unique ribosome recycling strategy by HflXr which is distinct from that mediated by RRF and EF-G. The resemblance between HflXr and housekeeping HflX suggests that the alternative ribosome recycling mechanism reported here is universal in the prokaryotic kingdom.