Summary information and primary citation
- PDB-id
- 8v26; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- cryo-EM (3.33 Å)
- Summary
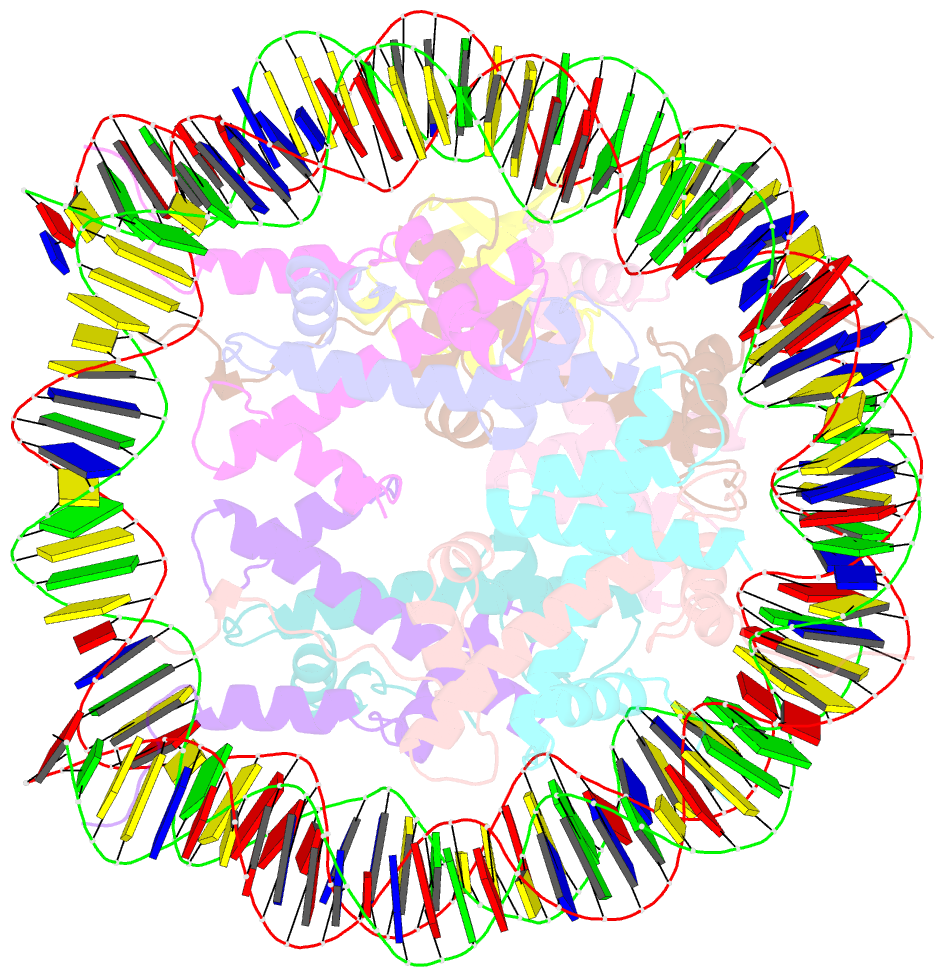
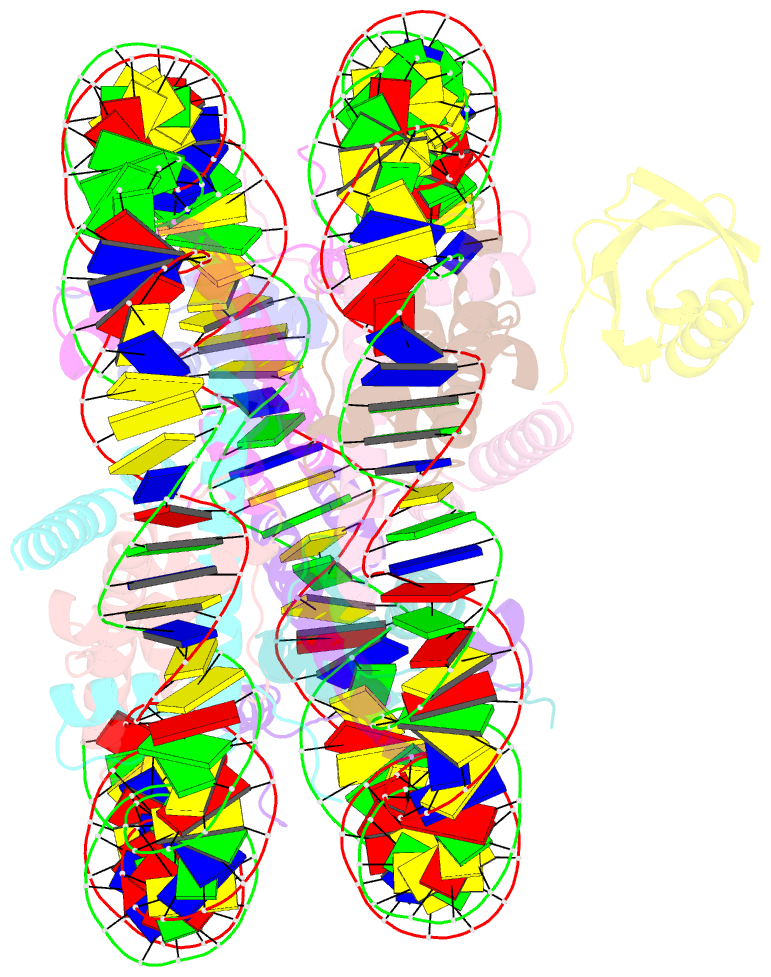
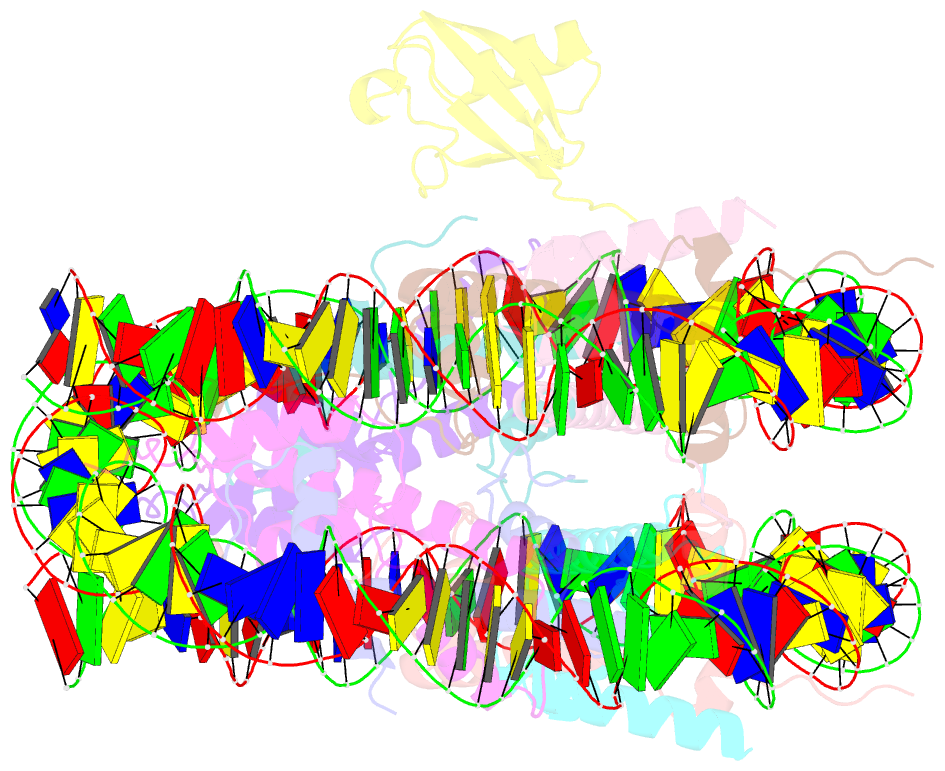
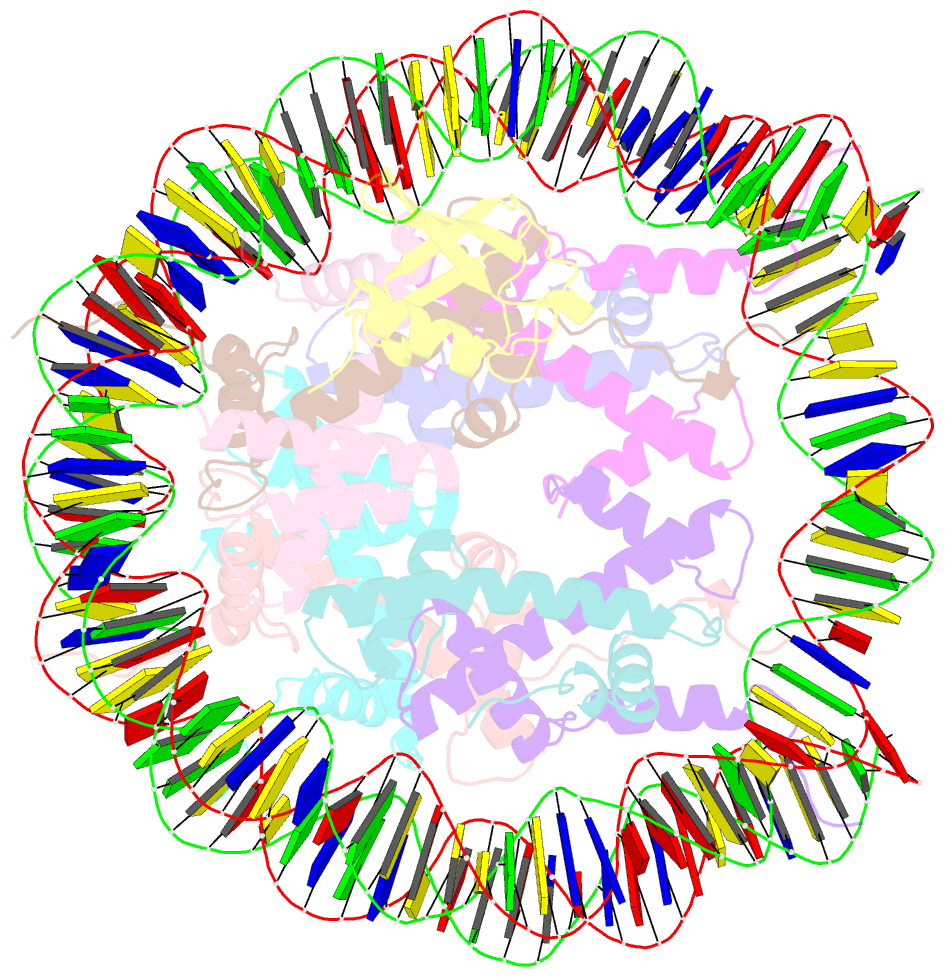
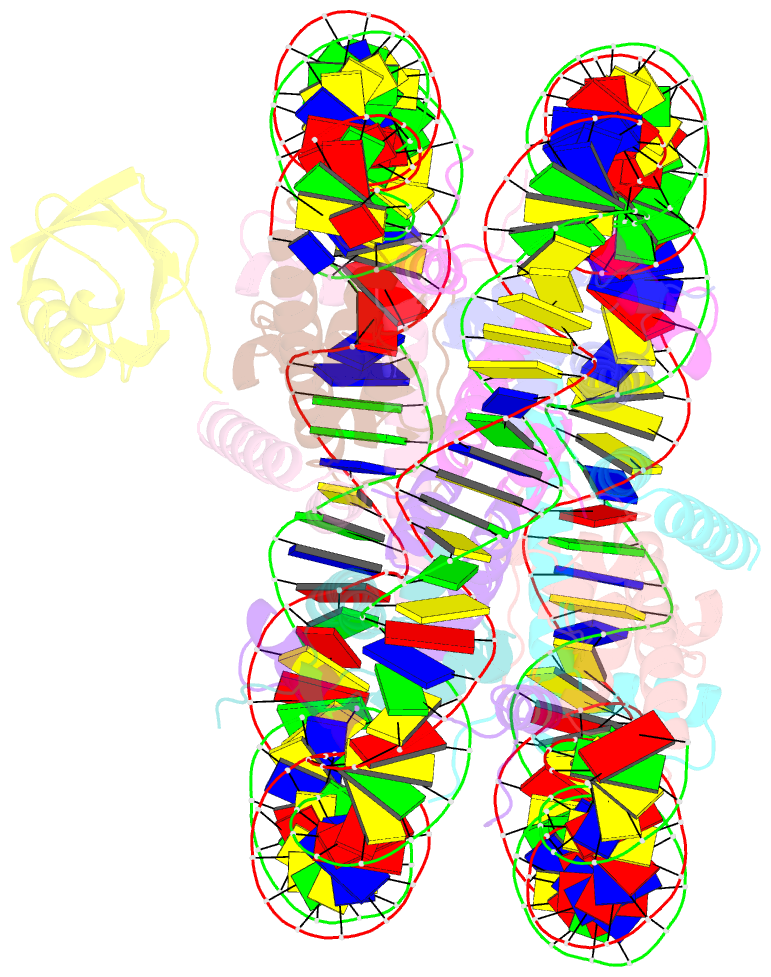
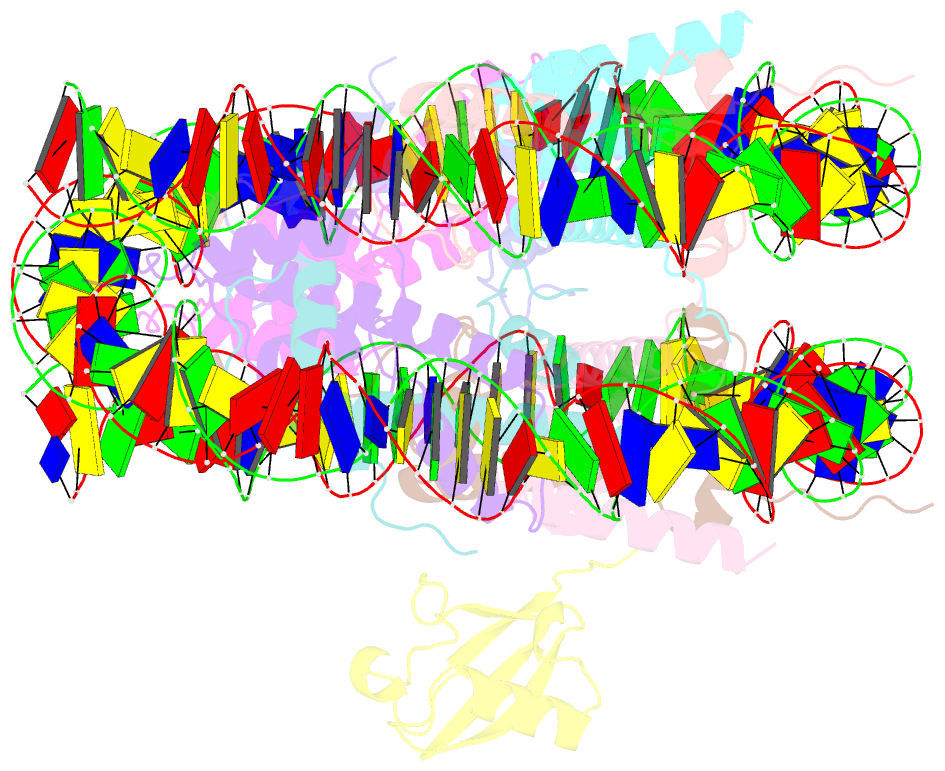
- H2bk120ub-modified nucleosome ubiquitin position 2
- Reference
- Hicks CW, Rahman S, Gloor SL, Fields JK, Husby NL, Vaidya A, Maier KE, Morgan M, Keogh MC, Wolberger C (2024): "Ubiquitinated histone H2B as gatekeeper of the nucleosome acidic patch." Nucleic Acids Res., 52, 9978-9995. doi: 10.1093/nar/gkae698.
- Abstract
- Monoubiquitination of histones H2B-K120 (H2BK120ub) and H2A-K119 (H2AK119ub) play opposing roles in regulating transcription and chromatin compaction. H2BK120ub is a hallmark of actively transcribed euchromatin, while H2AK119ub is highly enriched in transcriptionally repressed heterochromatin. Whereas H2BK120ub is known to stimulate the binding or activity of various chromatin-modifying enzymes, this post-translational modification (PTM) also interferes with the binding of several proteins to the nucleosome H2A/H2B acidic patch via an unknown mechanism. Here, we report cryoEM structures of an H2BK120ub nucleosome showing that ubiquitin adopts discrete positions that occlude the acidic patch. Molecular dynamics simulations show that ubiquitin remains stably positioned over this nucleosome region. By contrast, our cryoEM structures of H2AK119ub nucleosomes show ubiquitin adopting discrete positions that minimally occlude the acidic patch. Consistent with these observations, H2BK120ub, but not H2AK119ub, abrogates nucleosome interactions with acidic patch-binding proteins RCC1 and LANA, and single-domain antibodies specific to this region. Our results suggest a mechanism by which H2BK120ub serves as a gatekeeper to the acidic patch and point to distinct roles for histone H2AK119 and H2BK120 ubiquitination in regulating protein binding to nucleosomes.