Summary information and primary citation
- PDB-id
- 8wh6; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- cryo-EM (3.27 Å)
- Summary
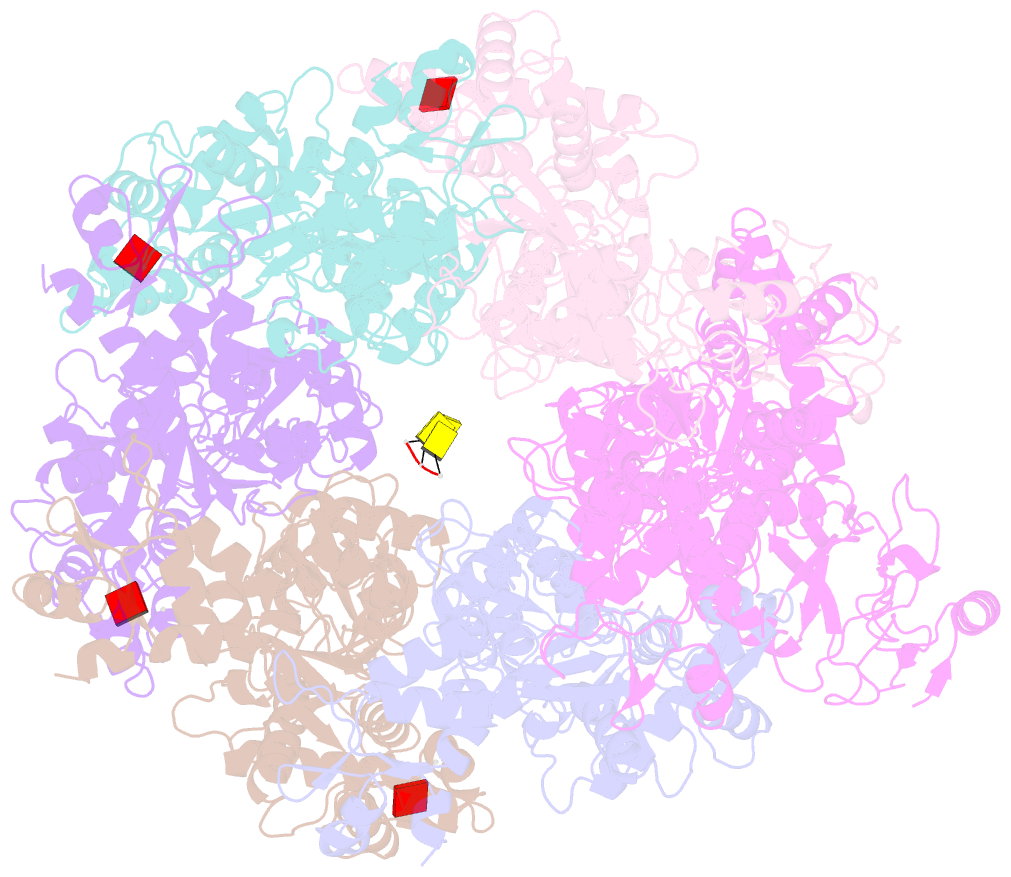
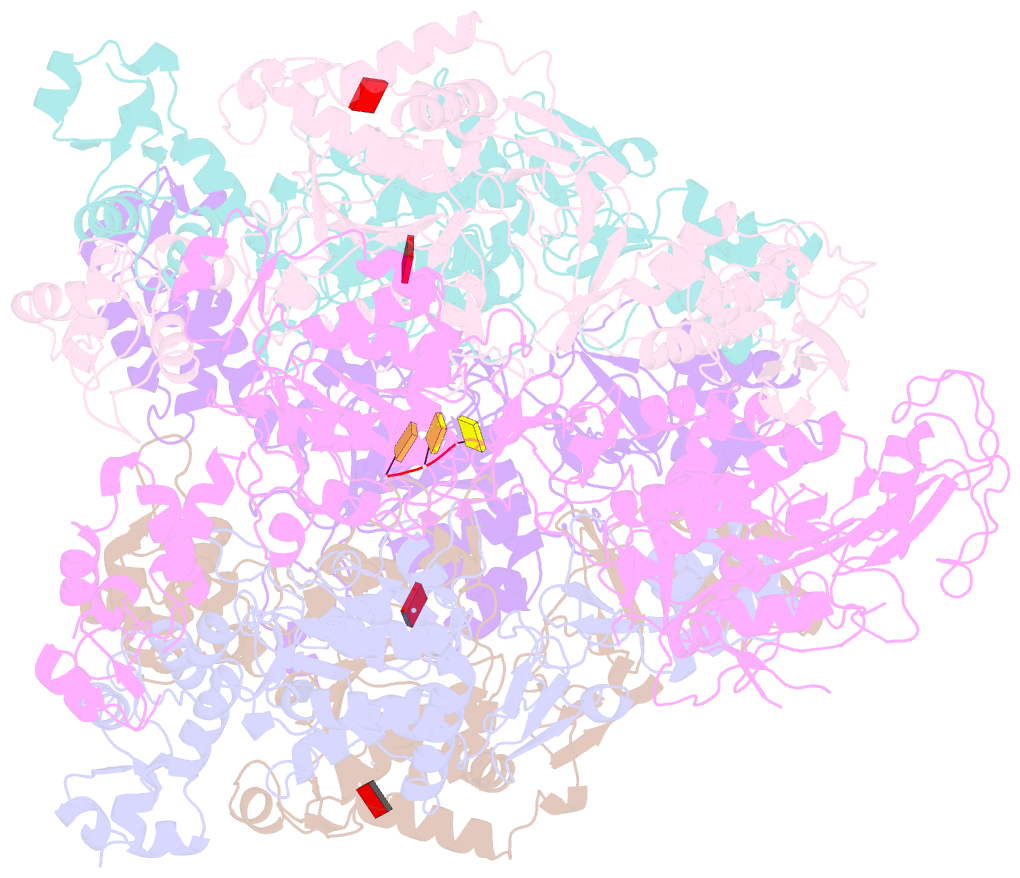
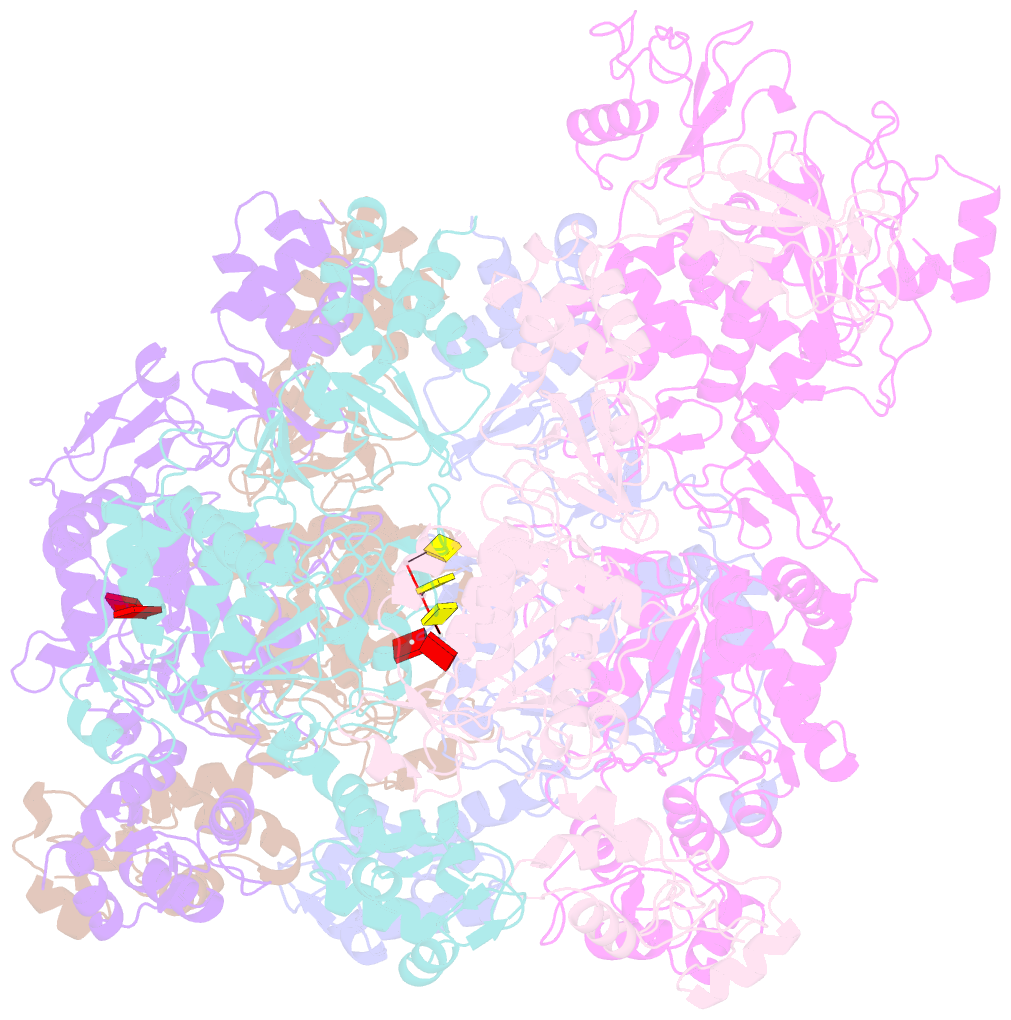
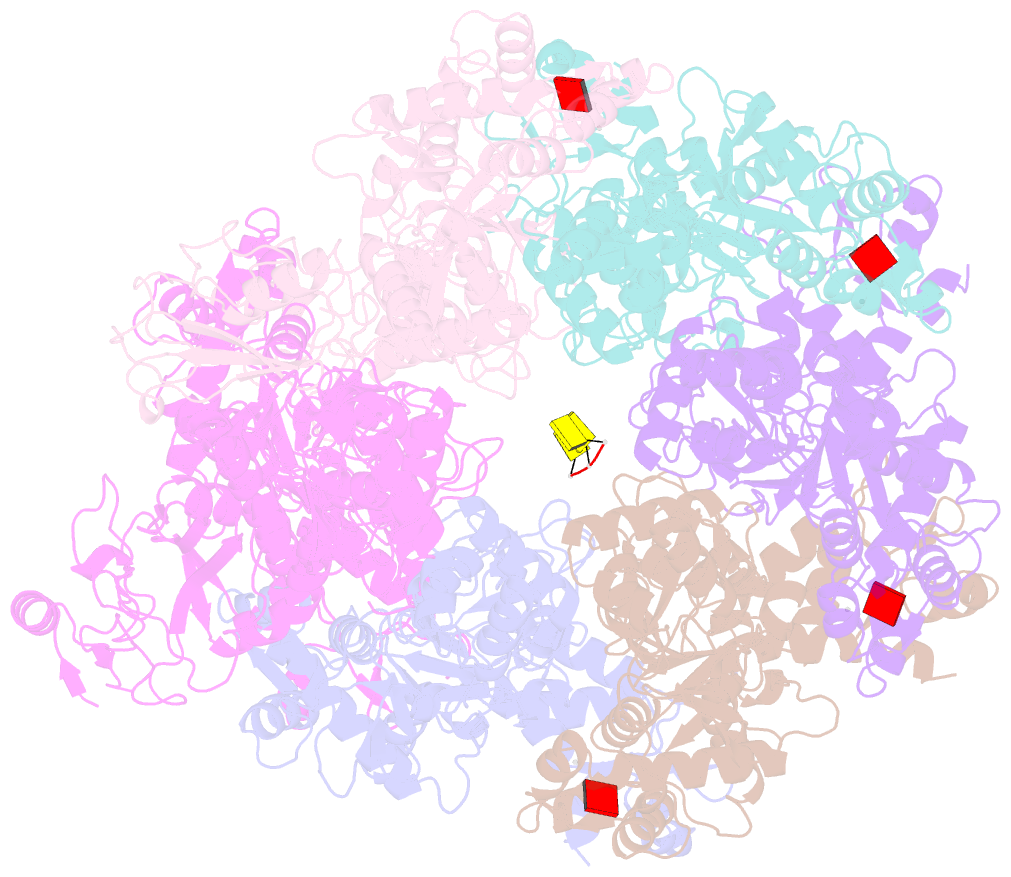
- Mpox e5 hexamer adp and ssDNA bound and clear primase domain conformation
- Reference
- Xu Y, Wu Y, Zhang Y, Gao K, Wu X, Yang Y, Li D, Yang B, Zhang Z, Dong C (2024): "Essential and multifunctional mpox virus E5 helicase-primase in double and single hexamer." Sci Adv, 10, eadl1150. doi: 10.1126/sciadv.adl1150.
- Abstract
- An outbreak of mpox virus in May 2022 has spread over 110 nonpandemic regions in the world, posing a great threat to global health. Mpox virus E5, a helicase-primase, plays an essential role in DNA replication, but the molecular mechanisms are elusive. Here, we report seven structures of mpox virus E5 in a double hexamer (DH) and six in single hexamer in different conformations, indicating a rotation mechanism for helicase and a coupling action for primase. The DH is formed through the interface of zinc-binding domains, and the central channel density indicates potential double-stranded DNA (dsDNA), which helps to identify dsDNA binding residues Arg249, Lys286, Lys315, and Lys317. Our work is important not only for understanding poxviral DNA replication but also for the development of novel therapeutics for serious poxviral infections including smallpox virus and mpox virus.