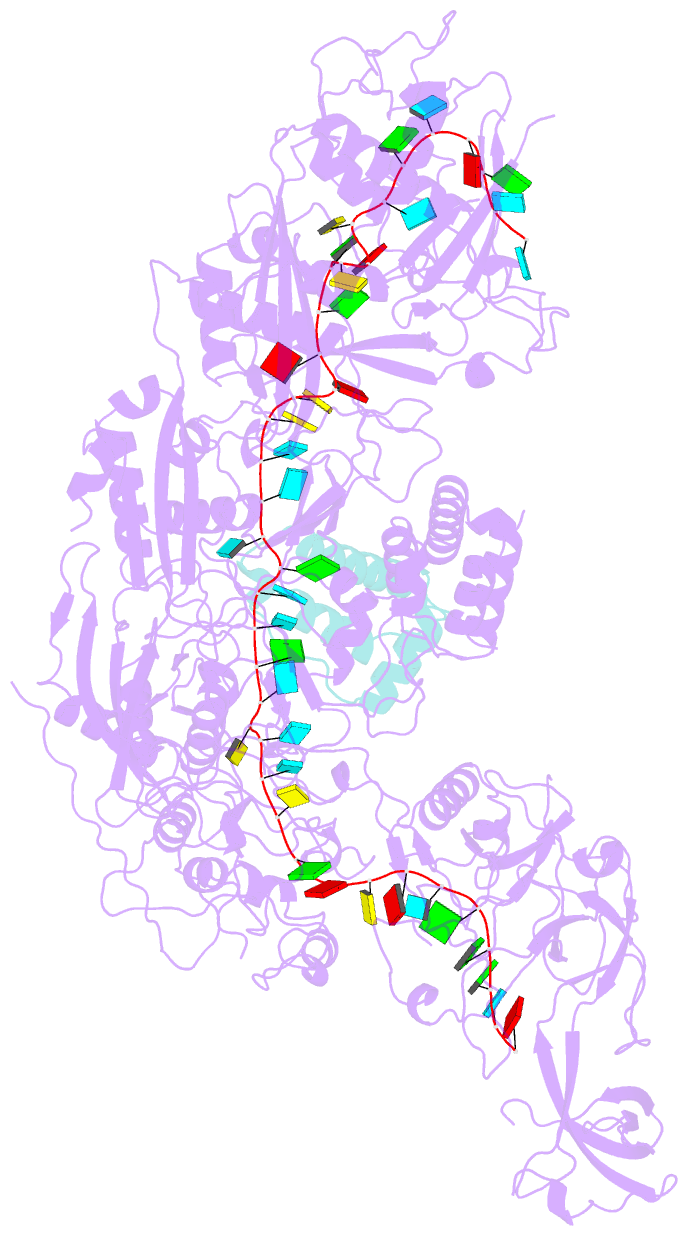
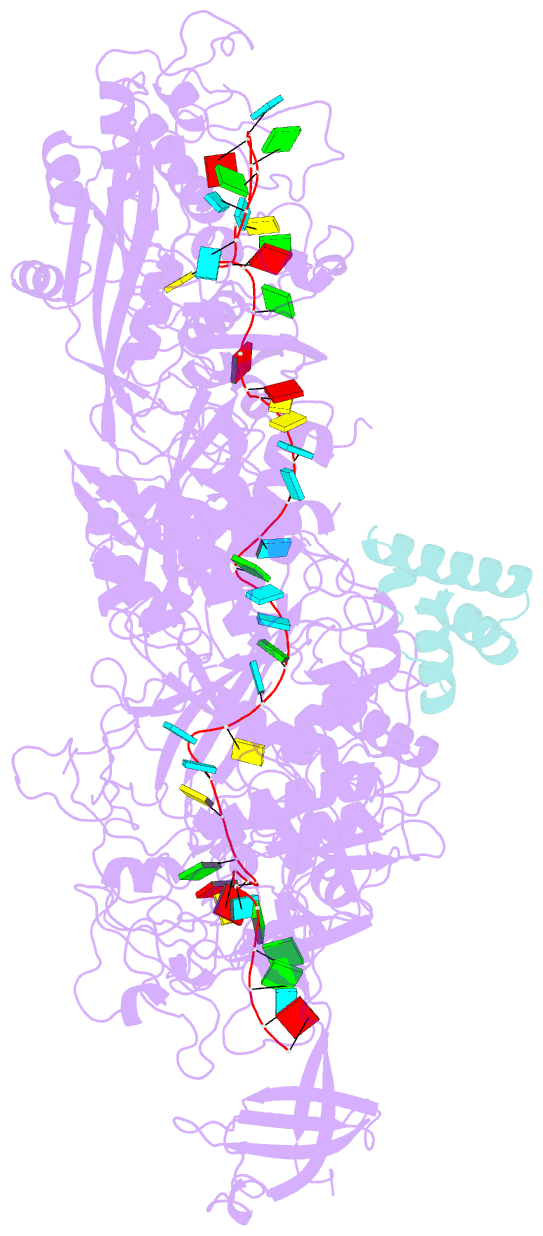
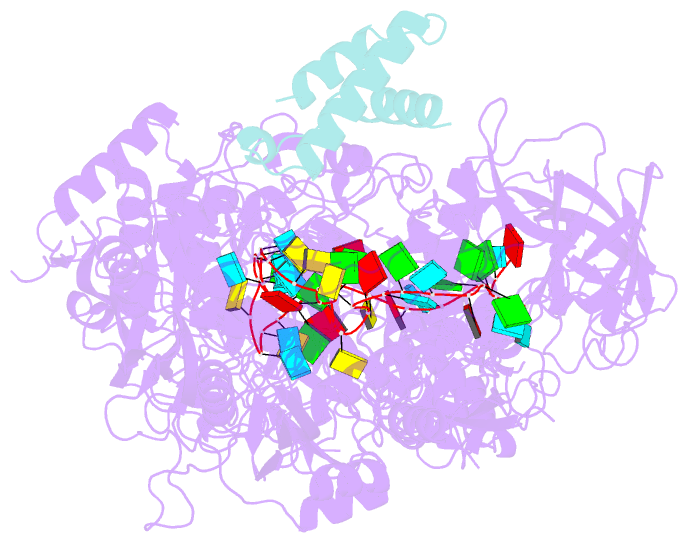
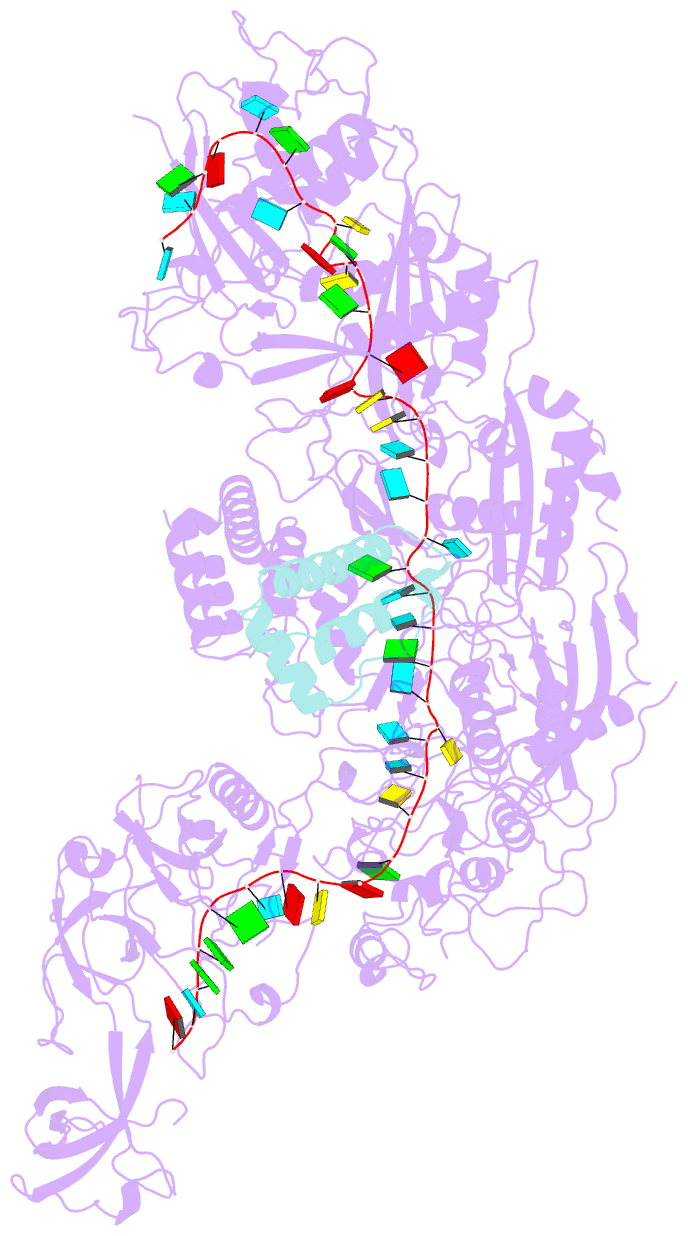
Summary information and primary citation
- PDB-id
- 8wml; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- immune system
- Method
- cryo-EM (2.86 Å)
- Summary
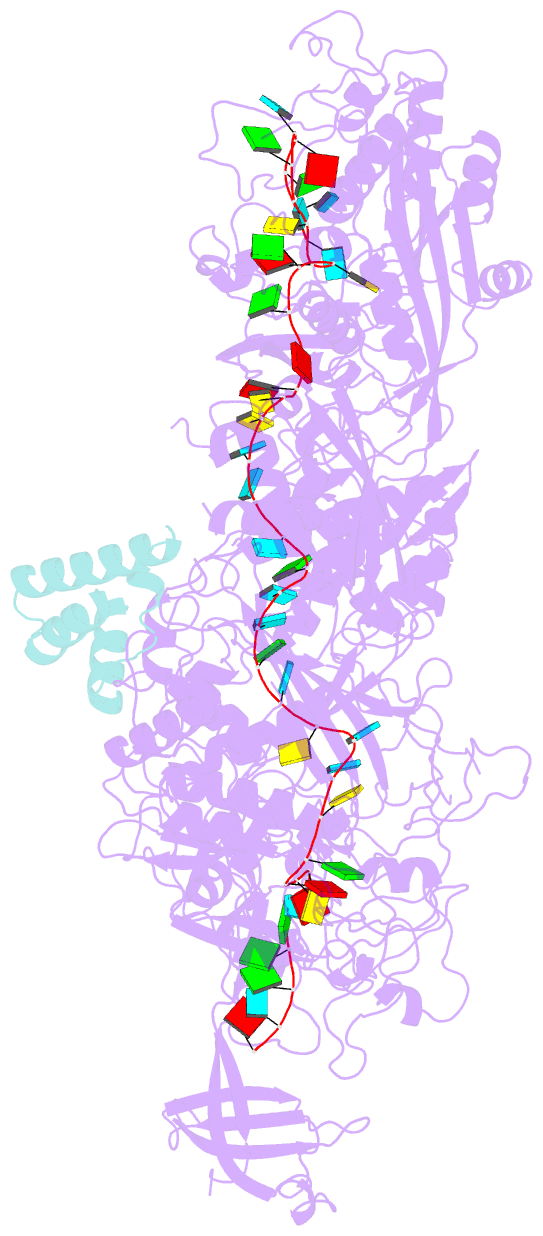
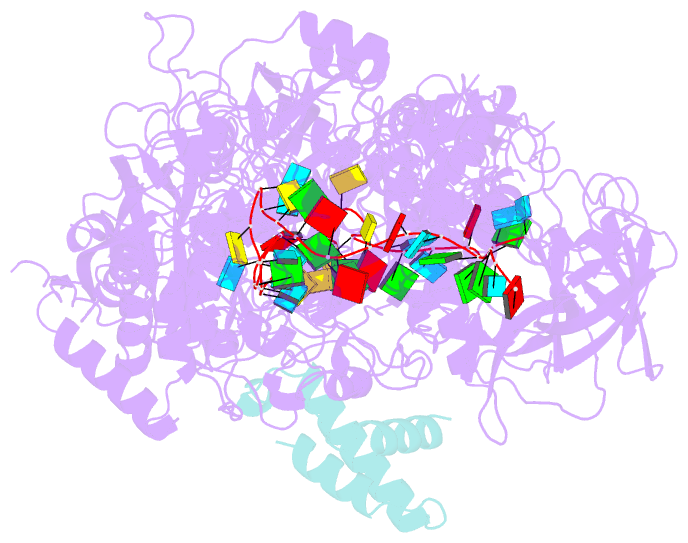
- cryo-EM structure of cas7-11-crrna bound to n-terminal of tpr-chat
- Reference
- Hong T, Luo Q, Ma H, Wang X, Li X, Shen C, Pang J, Wang Y, Chen Y, Zhang C, Su Z, Dong H, Tang X (2024): "Structural basis of negative regulation of CRISPR-Cas7-11 by TPR-CHAT." Signal Transduct Target Ther, 9, 111. doi: 10.1038/s41392-024-01821-4.
- Abstract
- CRISPR‒Cas7-11 is a Type III-E CRISPR-associated nuclease that functions as a potent RNA editing tool. Tetratrico-peptide repeat fused with Cas/HEF1-associated signal transducer (TPR-CHAT) acts as a regulatory protein that interacts with CRISPR RNA (crRNA)-bound Cas7-11 to form a CRISPR-guided caspase complex (Craspase). However, the precise modulation of Cas7-11's nuclease activity by TPR-CHAT to enhance its utility requires further study. Here, we report cryo-electron microscopy (cryo-EM) structures of Desulfonema ishimotonii (Di) Cas7-11-crRNA, complexed with or without the full length or the N-terminus of TPR-CHAT. These structures unveil the molecular features of the Craspase complex. Structural analysis, combined with in vitro nuclease assay and electrophoretic mobility shift assay, reveals that DiTPR-CHAT negatively regulates the activity of DiCas7-11 by preventing target RNA from binding through the N-terminal 65 amino acids of DiTPR-CHAT (DiTPR-CHATNTD). Our work demonstrates that DiTPR-CHATNTD can function as a small unit of DiCas7-11 regulator, potentially enabling safe applications to prevent overcutting and off-target effects of the CRISPR‒Cas7-11 system.