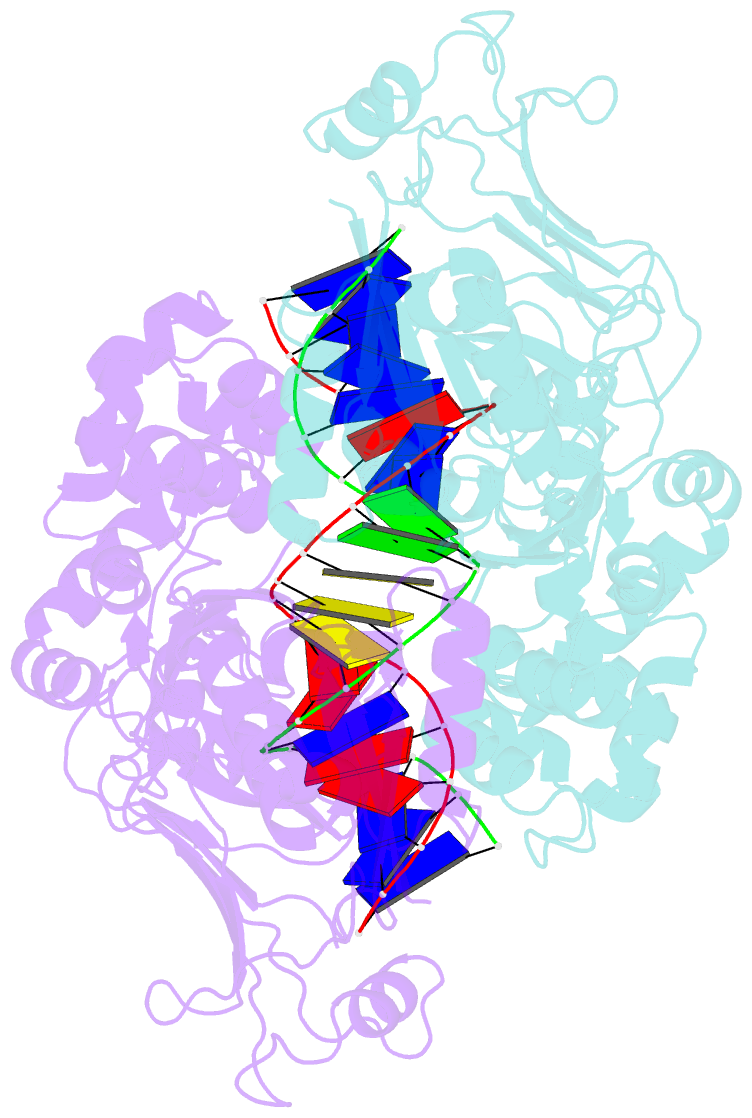
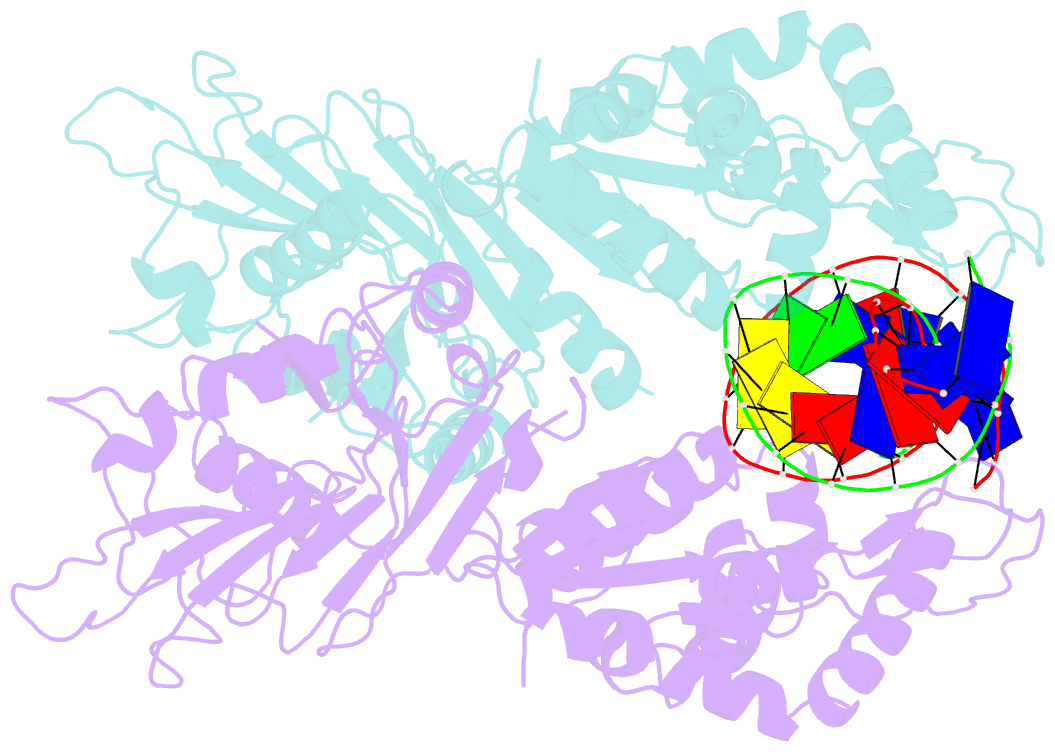
Summary information and primary citation
- PDB-id
- 8x51; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- cryo-EM (2.92 Å)
- Summary
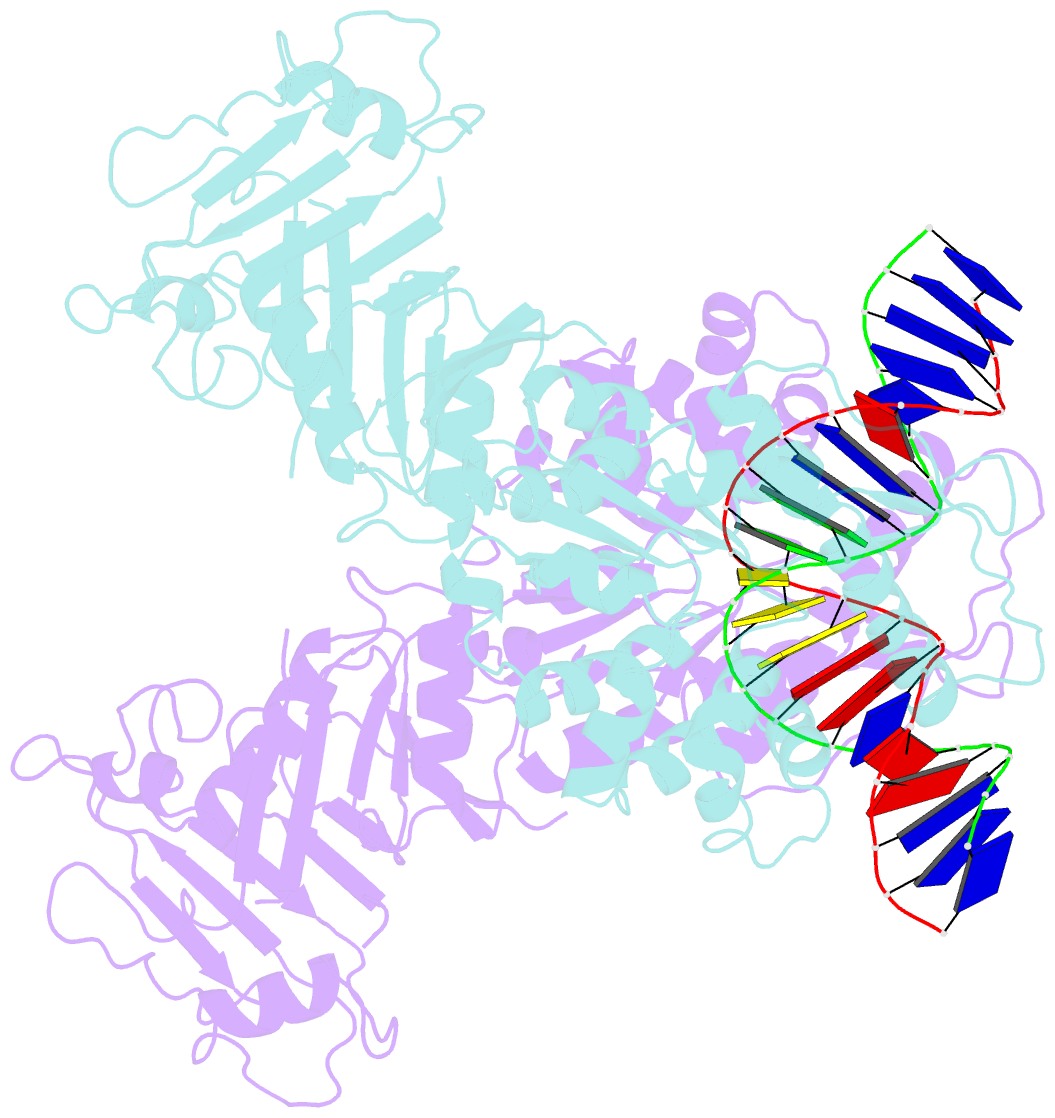
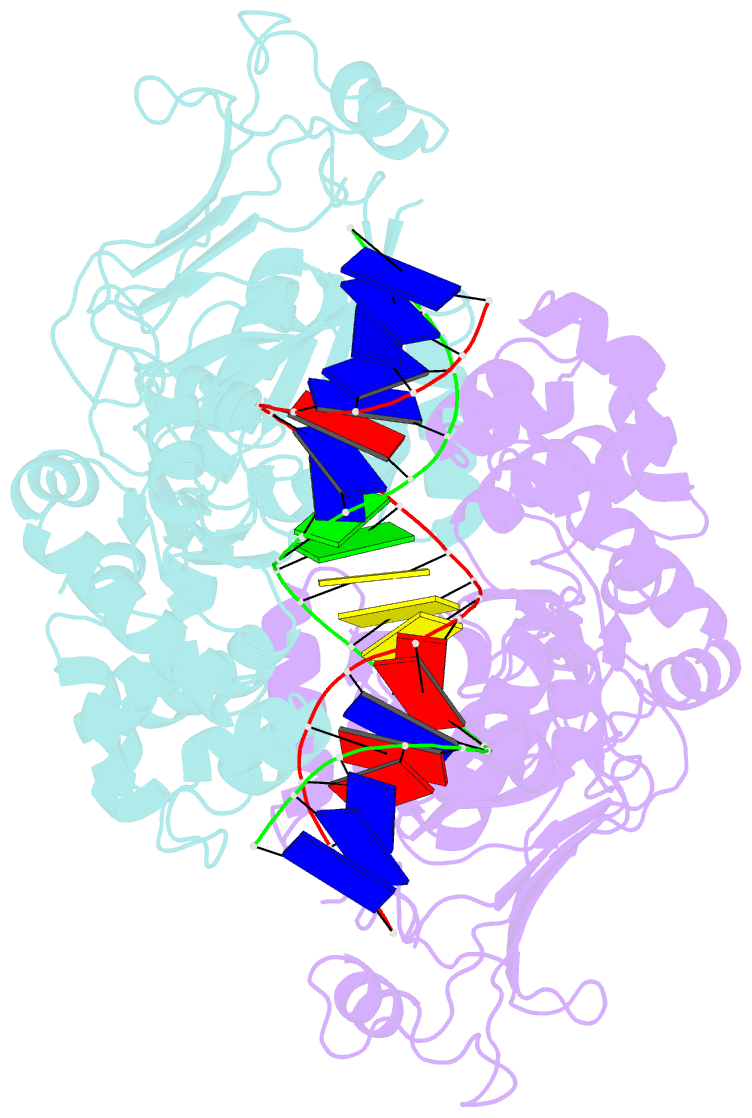
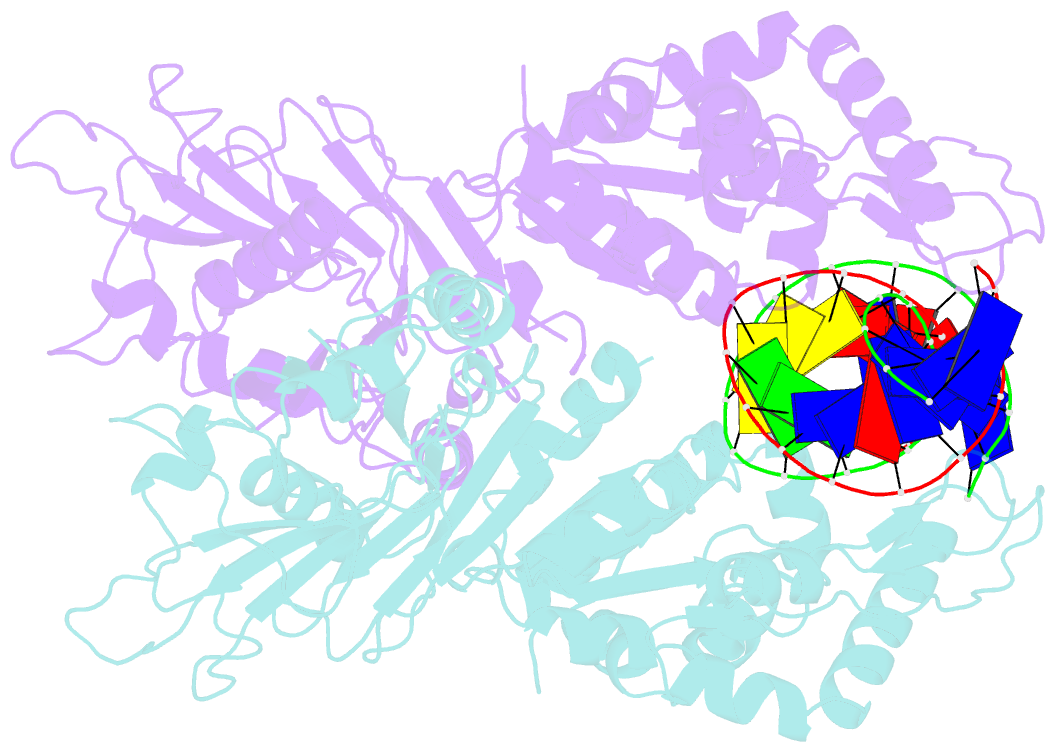
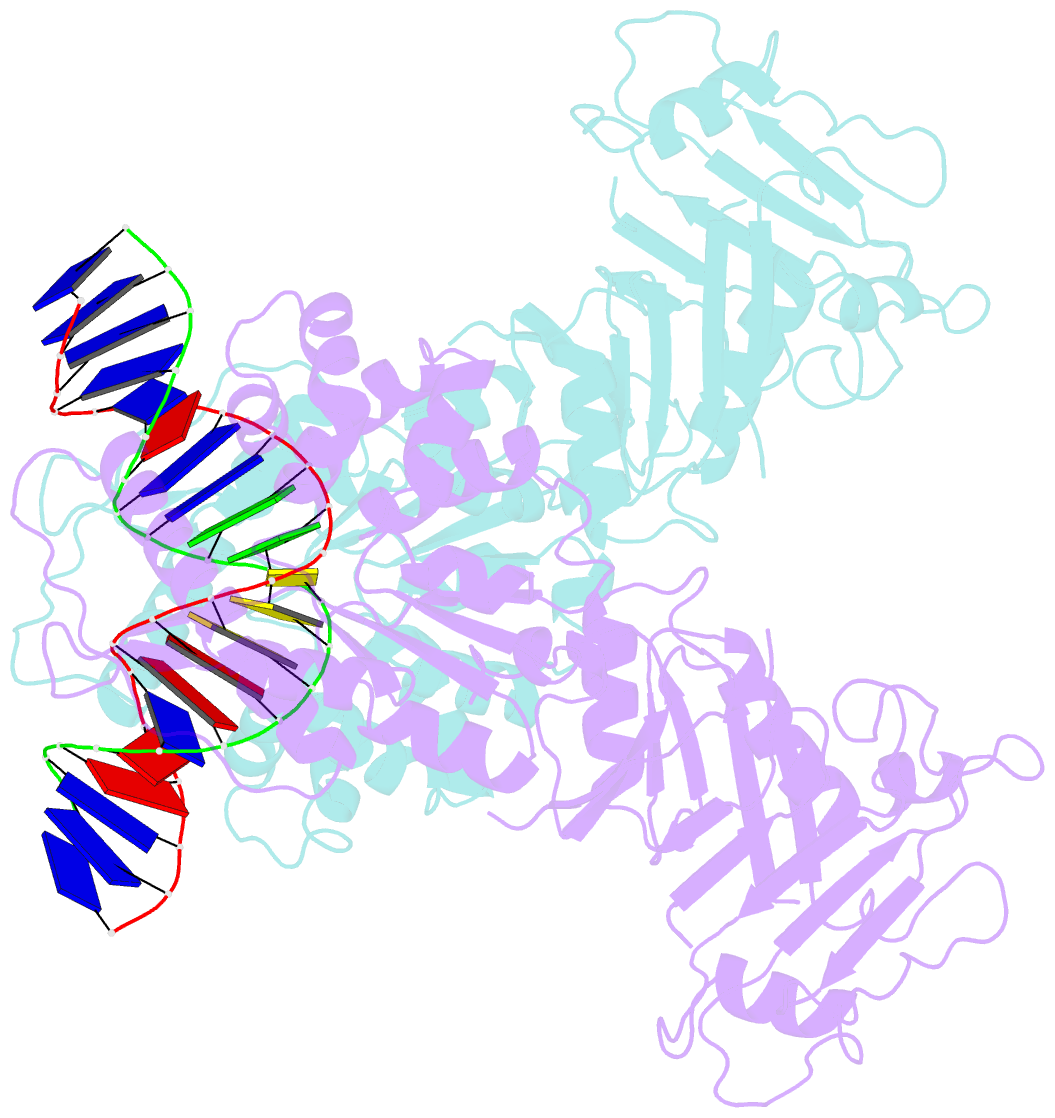
- Structure of DNA-bound gaja dimer (focused refinement)
- Reference
- Li J, Cheng R, Wang Z, Yuan W, Xiao J, Zhao X, Du X, Xia S, Wang L, Zhu B, Wang L (2024): "Structures and activation mechanism of the Gabija anti-phage system." Nature, 629, 467-473. doi: 10.1038/s41586-024-07270-x.
- Abstract
- Prokaryotes have evolved intricate innate immune systems against phage infection1-7. Gabija is a highly abundant prokaryotic defense system consisting of two components, GajA and GajB8. We previously demonstrated that GajA functions as a DNA endonuclease that is inactive in the presence of ATP9. To reveal how the Gabija system is activated for anti-phage defense, we report its cryo-electron microscopy (cryo-EM) structures in five states, including apo GajA, GajA in complex with DNA, GajA bound by ATP, apo GajA-GajB, and GajA-GajB in complex with ATP/Mg2+. GajA is a rhombus-shaped tetramer with its ATPase domain clustered at the center and the Toprim domain located peripherally. ATP binding at the ATPase domain stabilizes the insertion region within the ATPase domain, keeping the Toprim domain in a closed state. Upon ATP depletion by phages, the Toprim domain opens to bind and cleave the DNA substrate. GajB, which docks on GajA, is activated by the cleaved DNA, ultimately leading to prokaryotic cell death. Our study presents a mechanistic landscape of Gajiba activation.