Summary information and primary citation
- PDB-id
- 8yfi; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- X-ray (2.02 Å)
- Summary
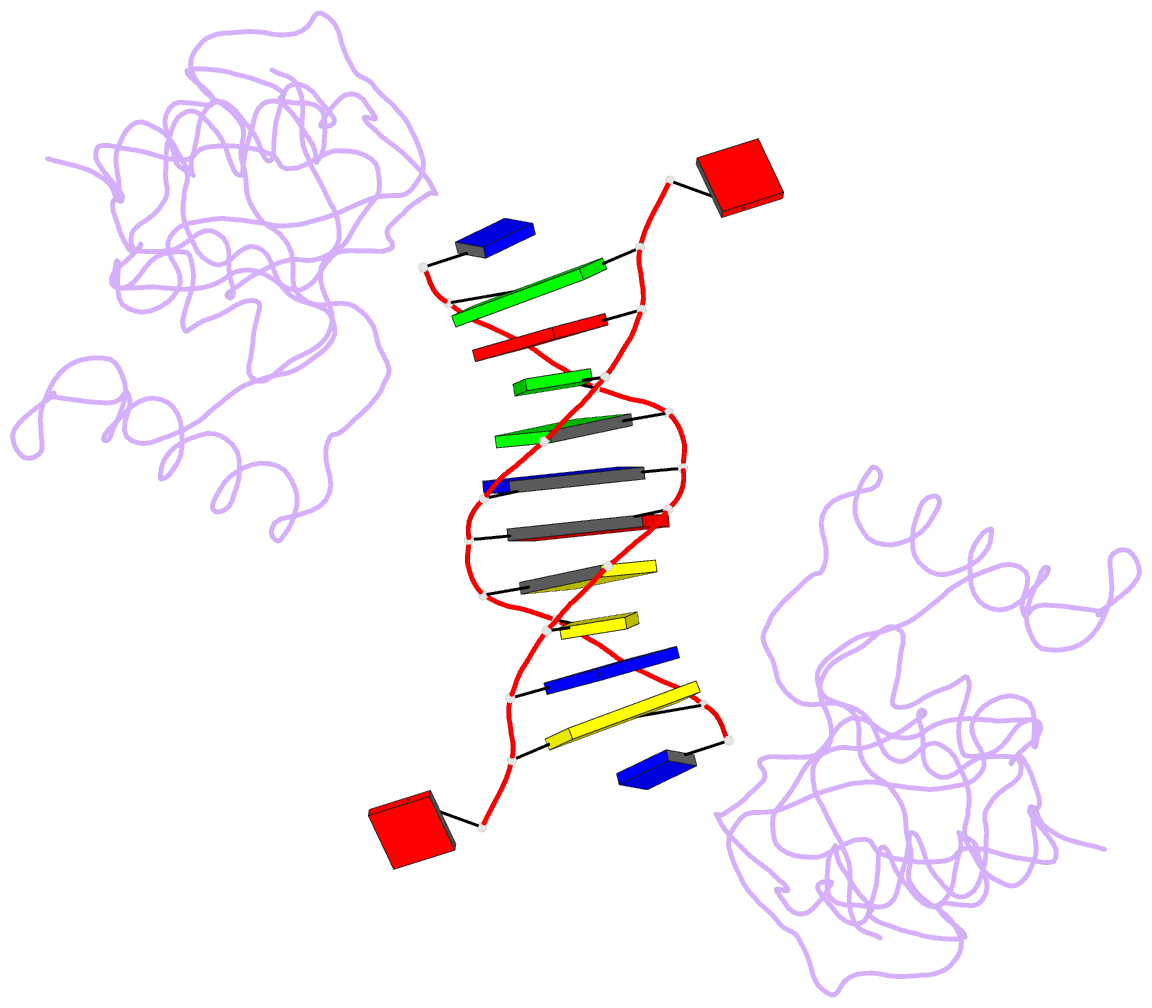
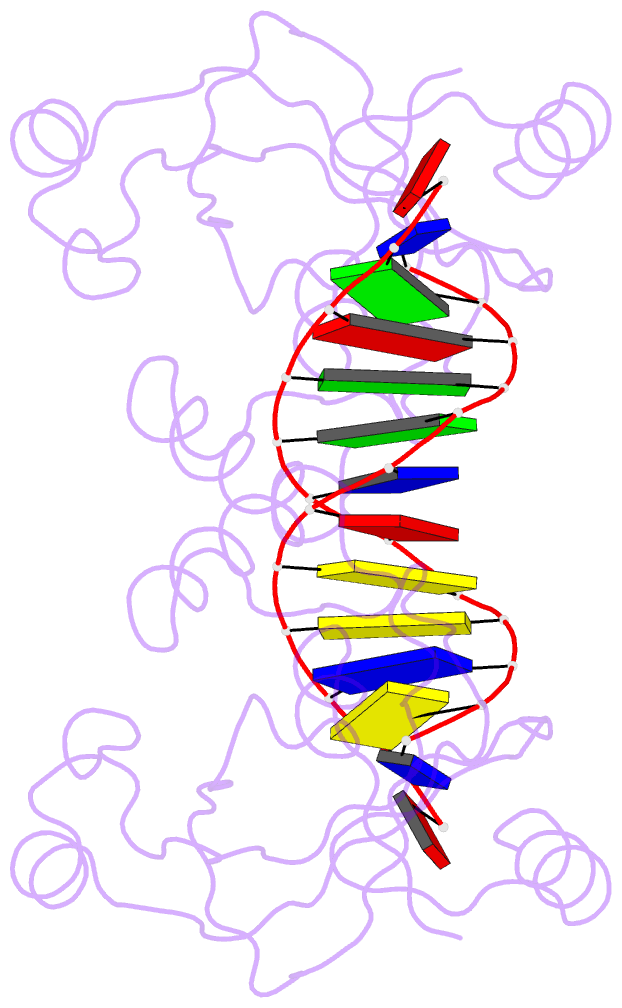
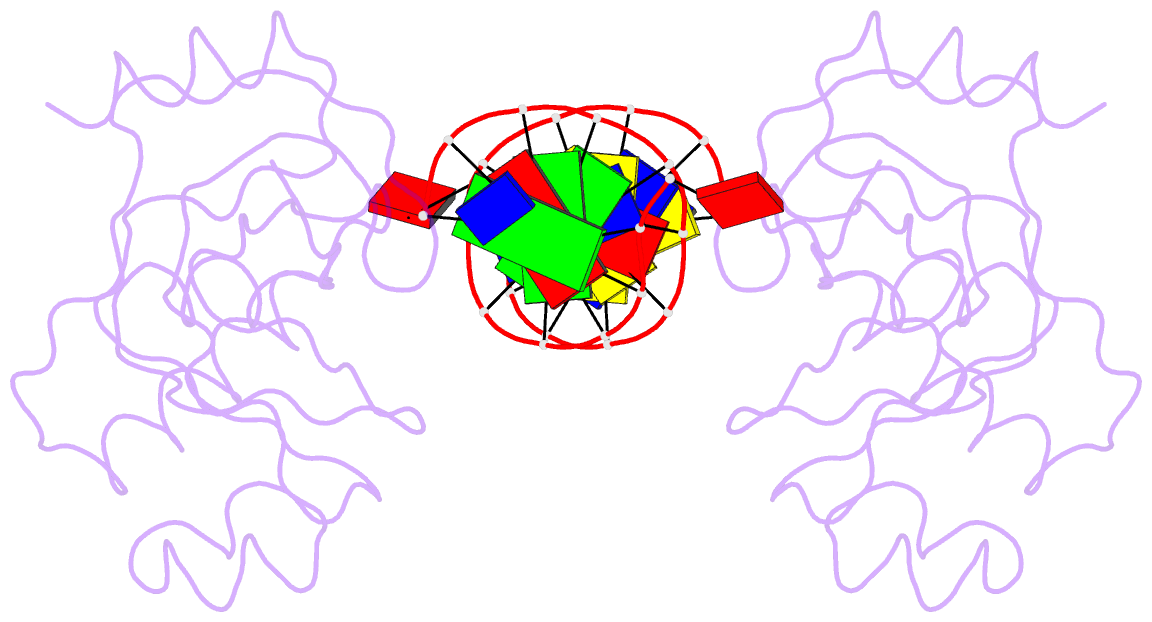
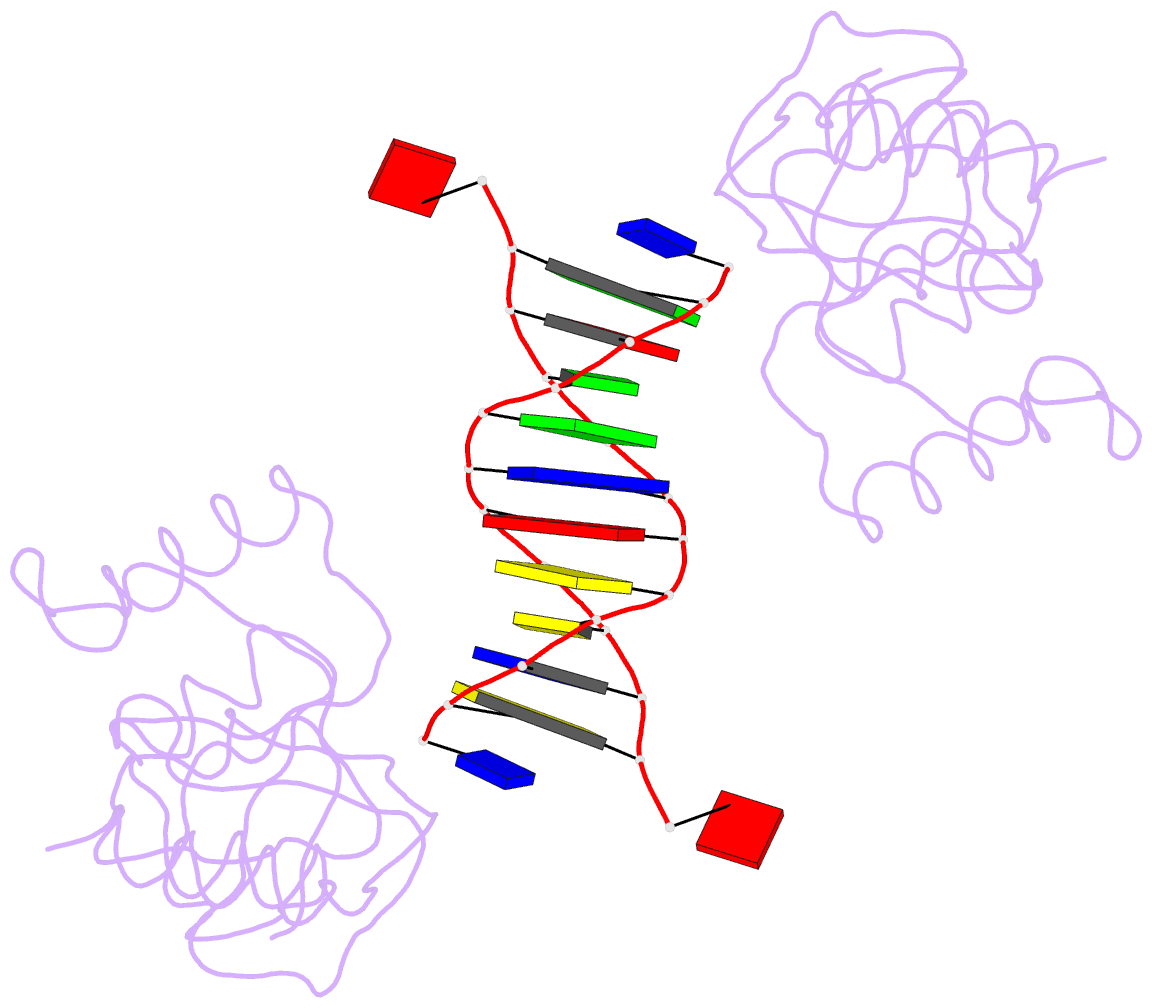
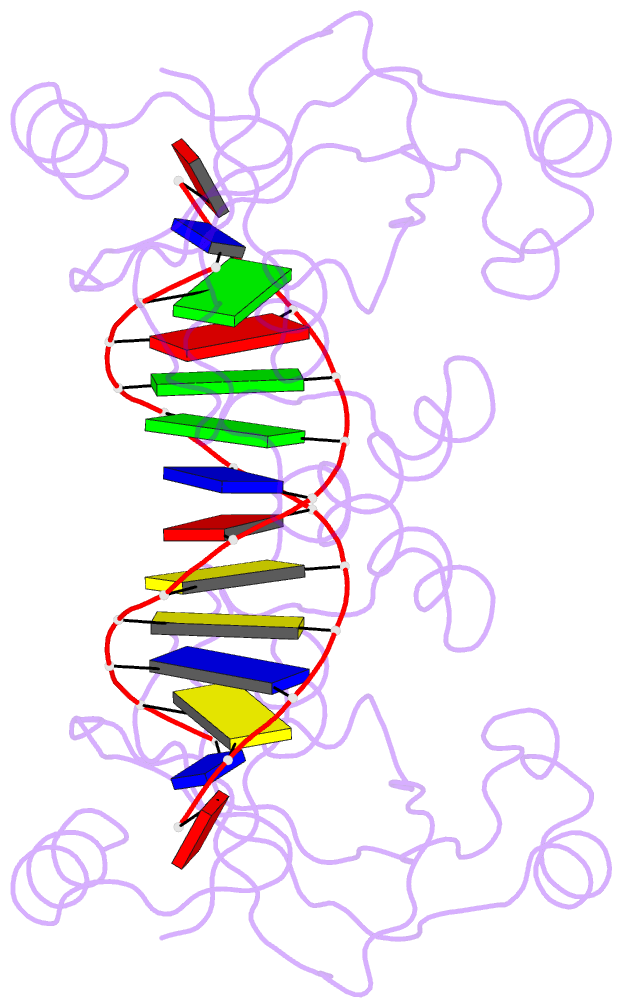
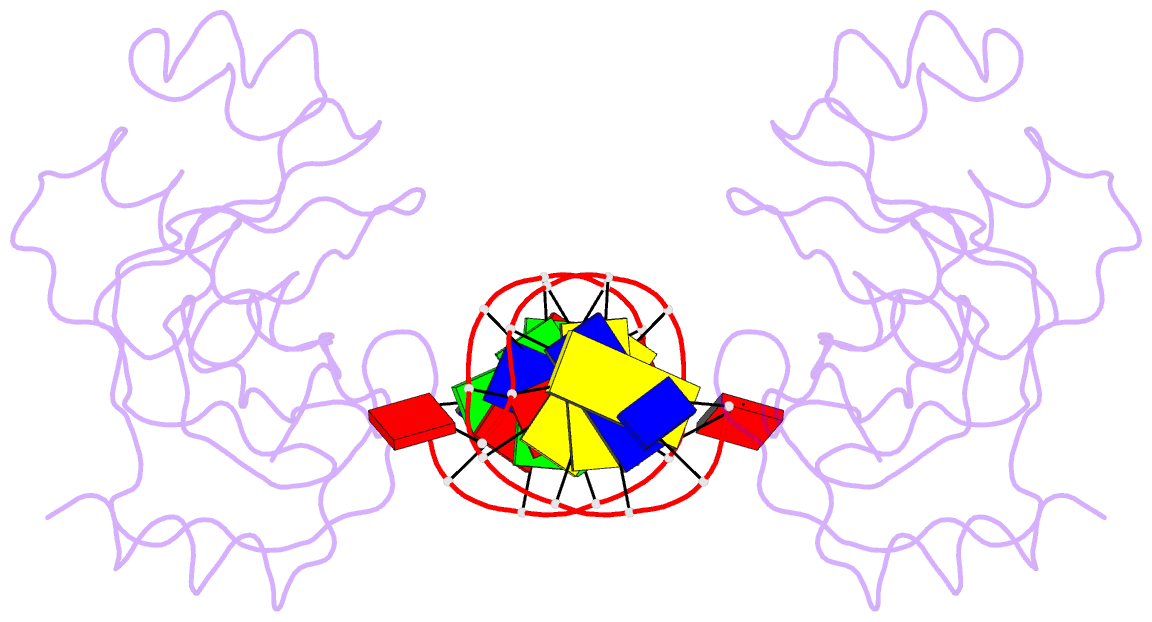
- Trip4 asch domain in complex with a 12bp dsDNA (5'-tgaggtacctca-3')
- Reference
- Hu C, Chen Z, Wang G, Yang H, Ding J (2024): "Biochemical and structural characterization of the DNA-binding properties of human TRIP4 ASCH domain reveals insights into its functional role." Structure, 32, 1208. doi: 10.1016/j.str.2024.05.012.
- Abstract
- TRIP4 is a conserved transcriptional coactivator that is involved in the regulation of the expression of multiple genes. It consists of a classical N-terminal C2HC5-like zinc-finger domain and a conserved C-terminal ASCH domain. Here, we characterized the DNA-binding properties of the human TRIP4 ASCH domain. Our biochemical data show that TRIP4-ASCH has comparable binding affinities toward ssDNA and dsDNA of different lengths, sequences, and structures. The crystal structures reveal that TRIP4-ASCH binds to DNA substrates in a sequence-independent manner through two adjacent positively charged surface patches: one binds to the 5'-end of DNA, and the other binds to the 3'-end of DNA. Further mutagenesis experiments and binding assays confirm the functional roles of key residues involved in DNA binding. In summary, our data demonstrate that TRIP4-ASCH binds to the 5' and 3'-ends of DNA in a sequence-independent manner, which will facilitate further studies of the biological function of TRIP4.