Summary information and primary citation
- PDB-id
- 8ymz; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA-DNA binding protein
- Method
- X-ray (2.95 Å)
- Summary
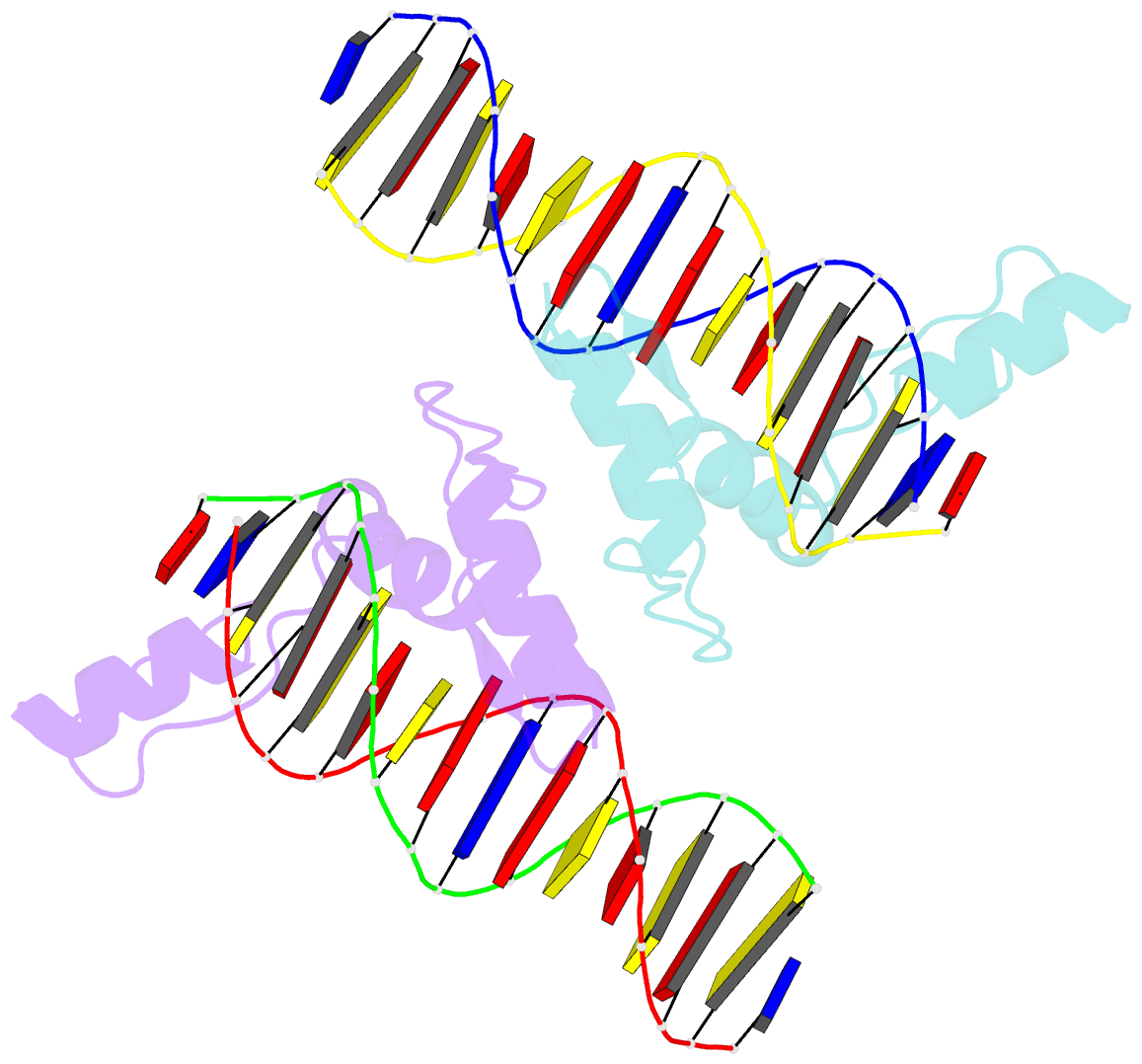
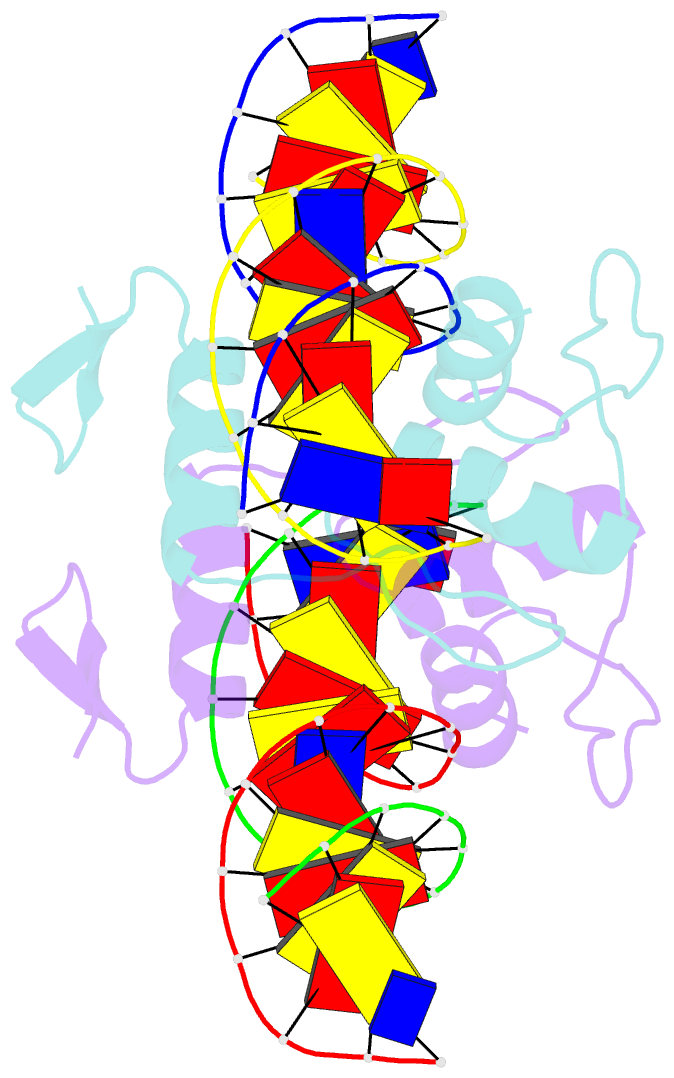
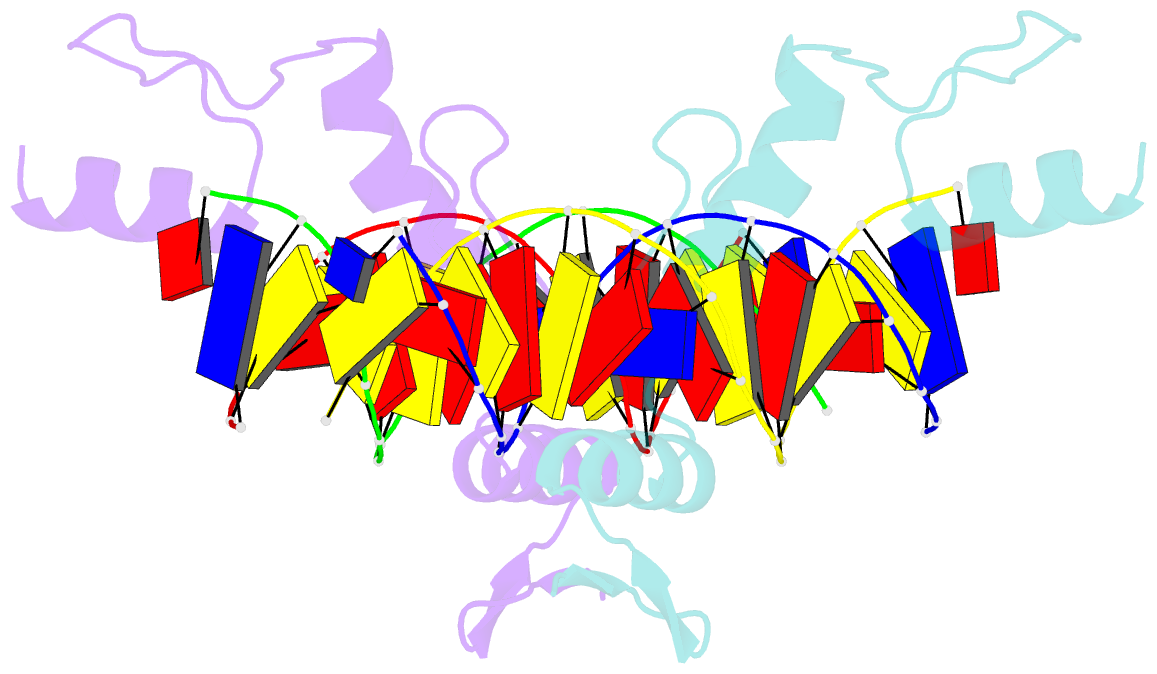
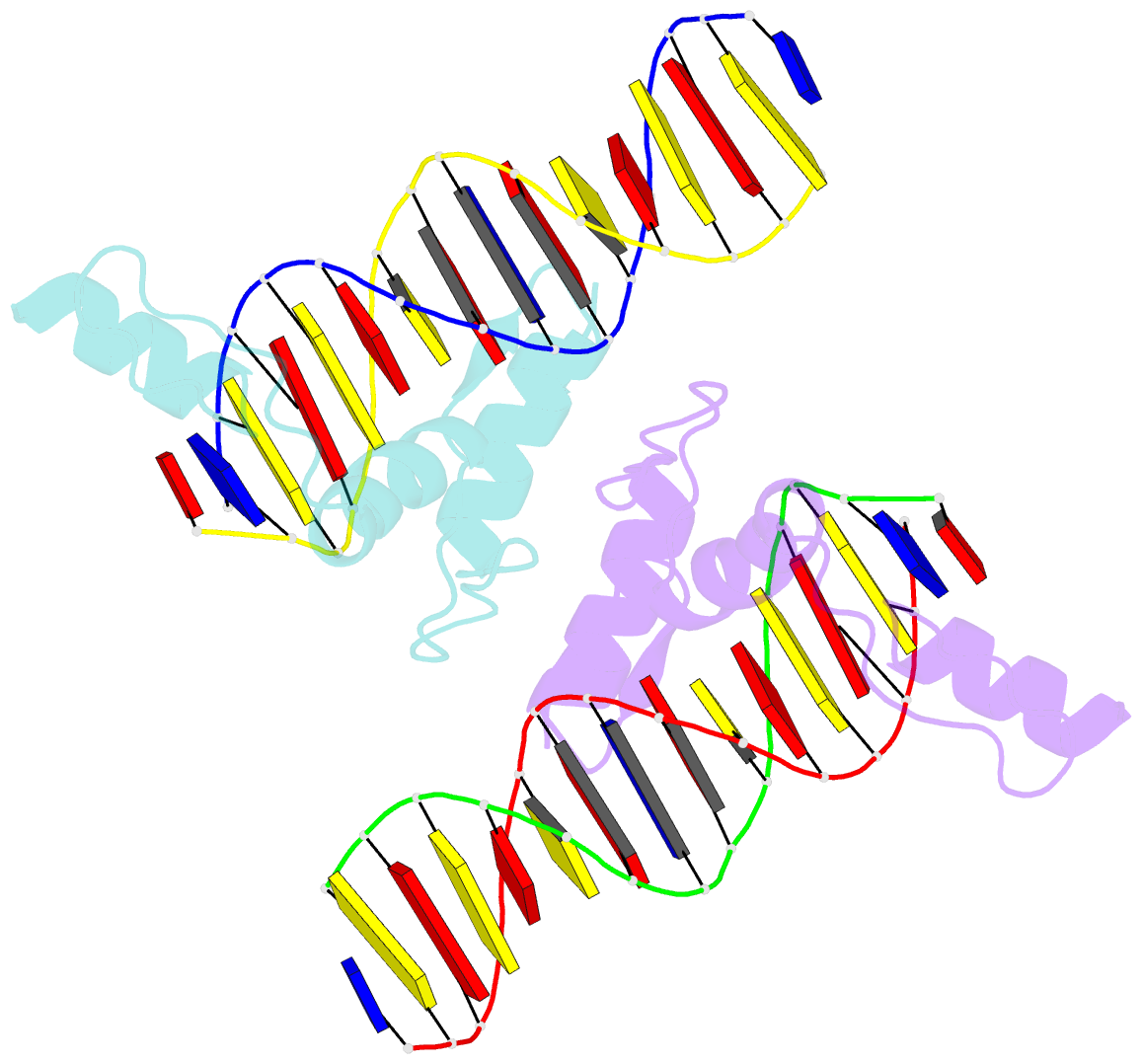
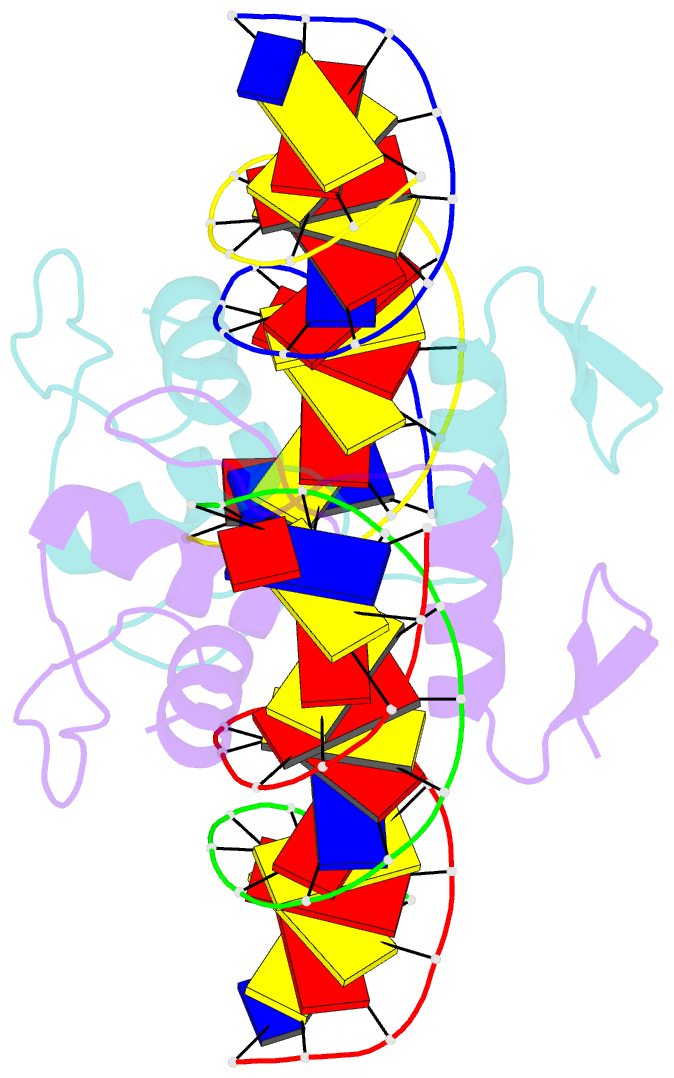
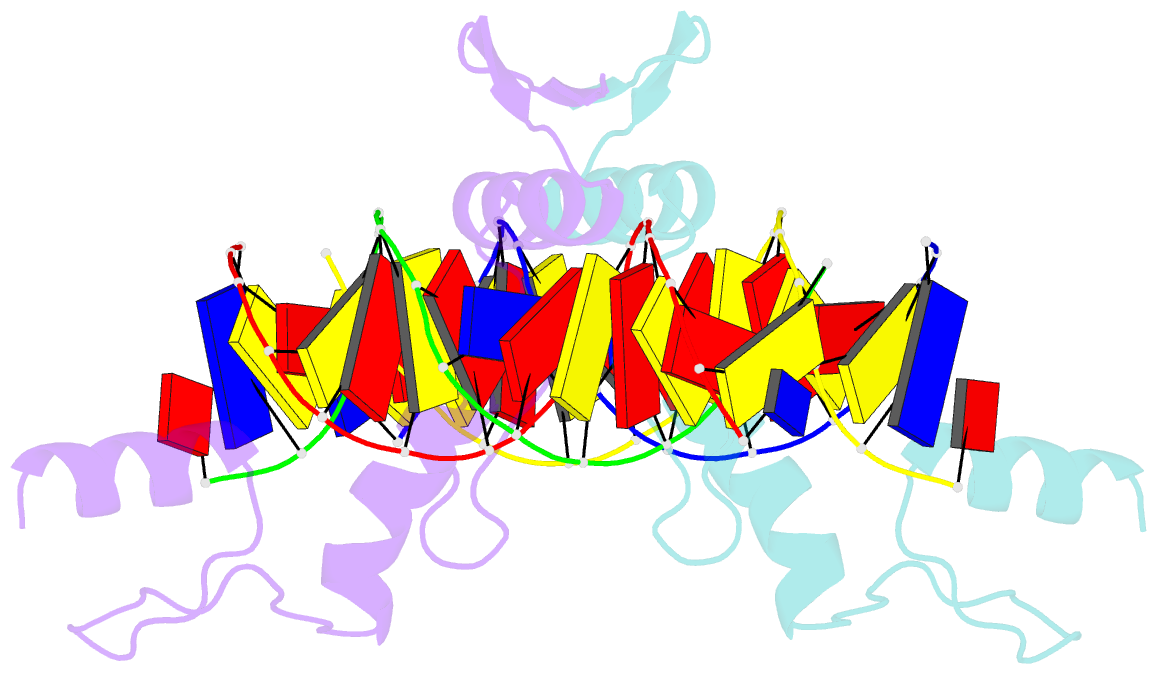
- Structure of zbtb43 in complex with caca containing b-form DNA
- Reference
- Yang Y, Zhang S, Xu L, Pan Y, Xuan Y, Kai Y, Chen X (2024): "Structural insights into the recognition of purine-pyrimidine dinucleotide repeats by zinc finger protein ZBTB43." Febs J. doi: 10.1111/febs.17286.
- Abstract
- Purine-pyrimidine repeats (PPRs) can form left-handed Z-form DNA and induce DNA double-strand breaks (DSBs), posing a risk for genomic rearrangements and cancer. The zinc finger (ZF) and BTB domain-containing protein 43 (ZBTB43) is a transcription factor containing two Cys2-His2 (C2H2) and one C3H1 zinc fingers and plays a crucial role in maintaining genomic and epigenomic integrity by converting mutagenic Z-form PPRs to the B-form in prospermatogonia. Despite its importance, the molecular mechanism underlying the recognition of PPRs by ZBTB43 remains elusive. In this study, we determined the X-ray crystal structure of the ZBTB43 ZF1-3 in complex with the B-form DNA containing the CA repeats sequence. The structure reveals that ZF1 and ZF2 primarily recognize the CACA sequence through specific hydrogen-bonding and van der Waals contacts via a quadruple center involving Arg389, Met411, His413, and His414. These interactions were further validated by fluorescence-based DNA-binding assays using mutated ZBTB43 variants. Our structural investigation provides valuable insights into the recognition mechanism of PPRs by ZBTB43 and suggests a potential role for ZBTB43 in the transformation of Z-DNA to B-DNA, contributing to the maintenance of genomic stability.