Summary information and primary citation
- PDB-id
- 9ash; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- cryo-EM (2.58 Å)
- Summary
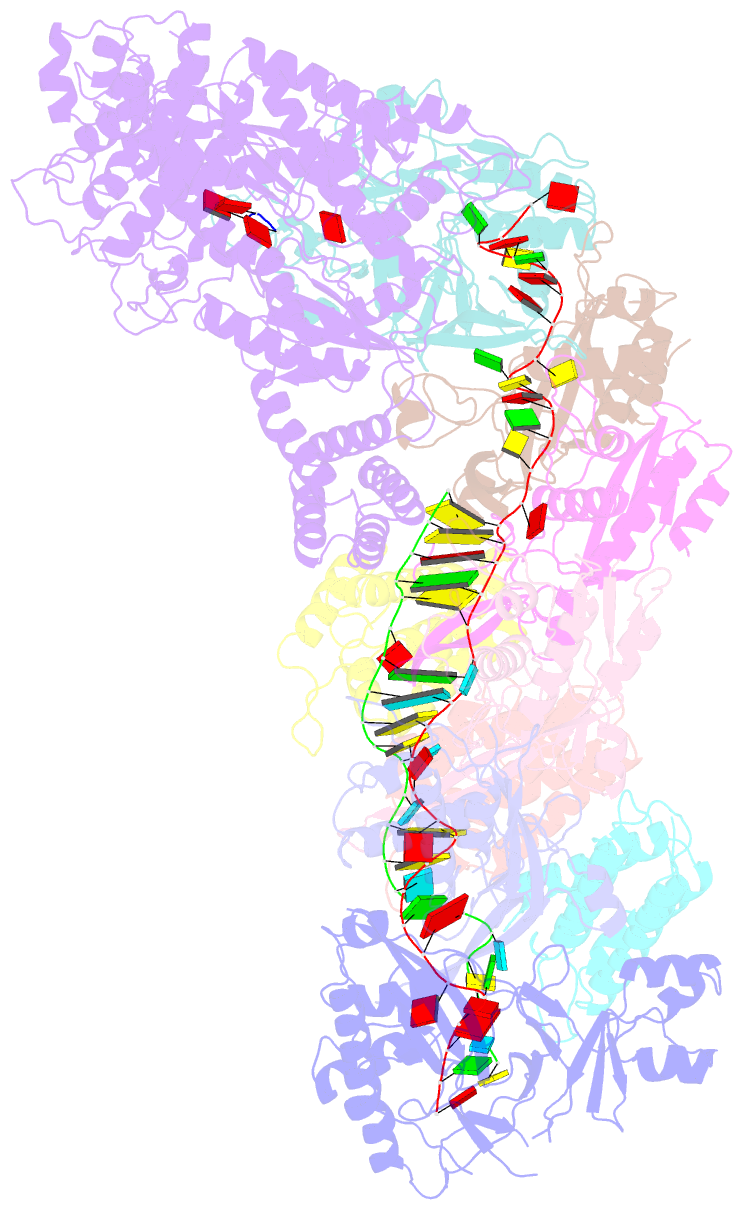
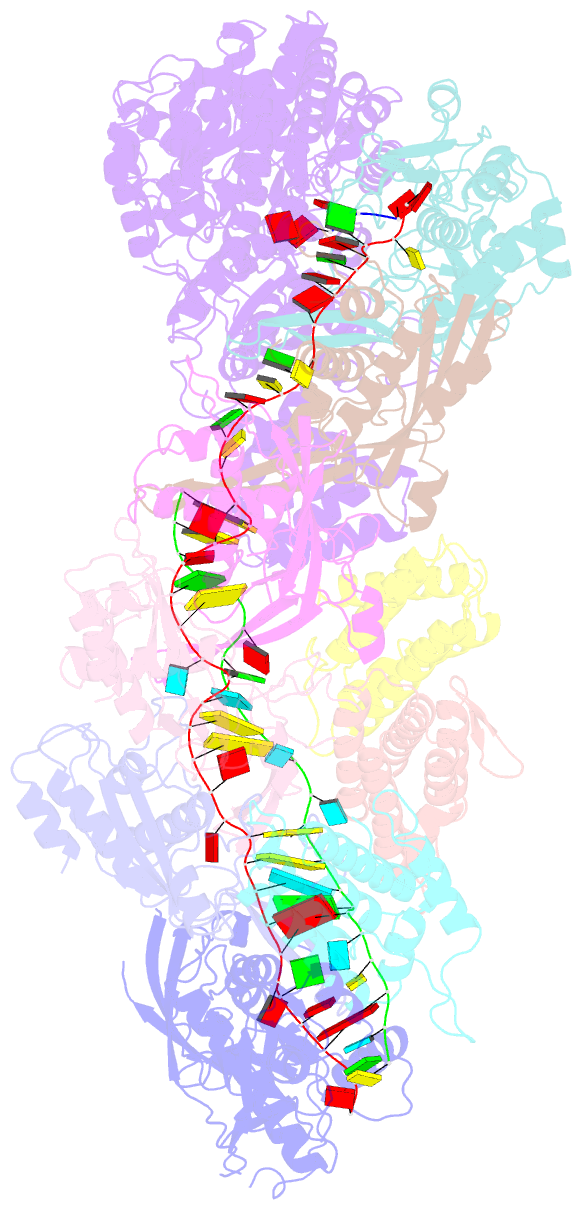
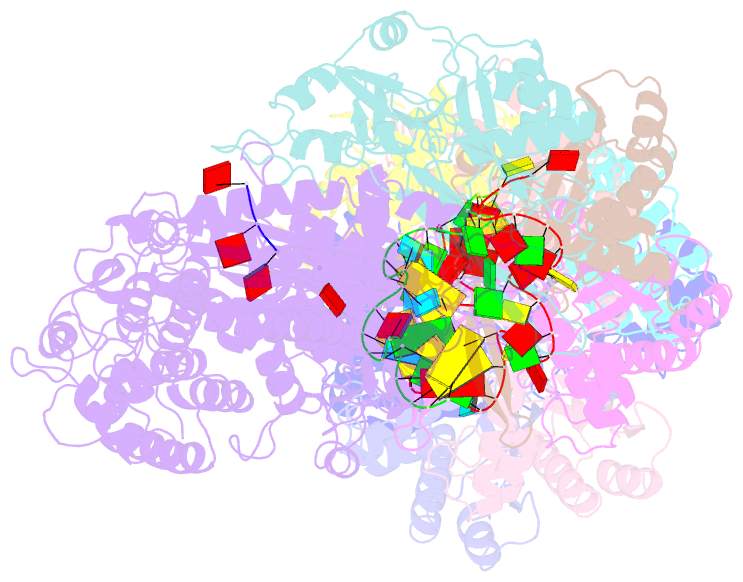
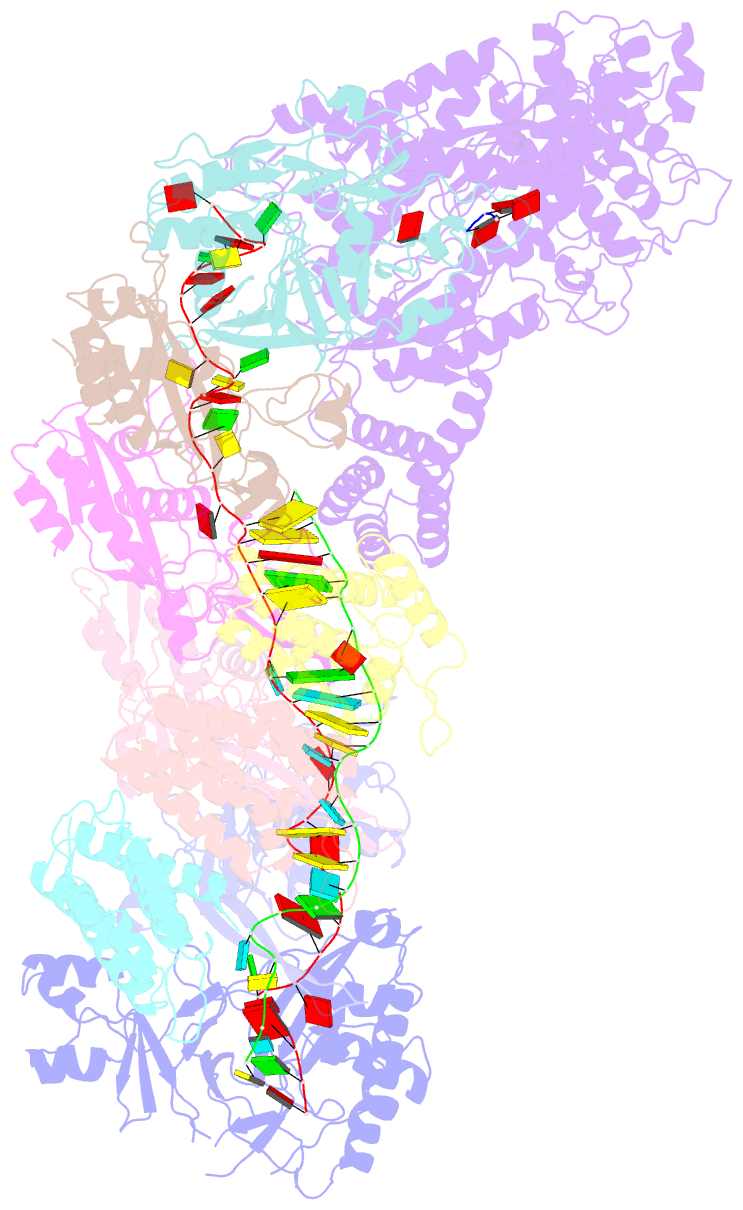
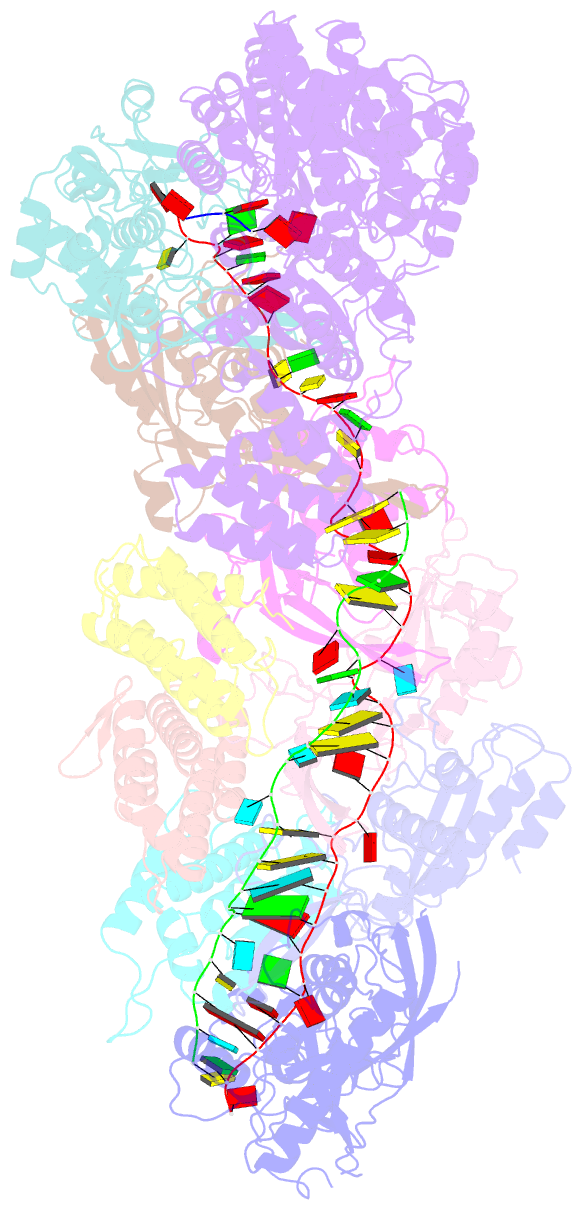
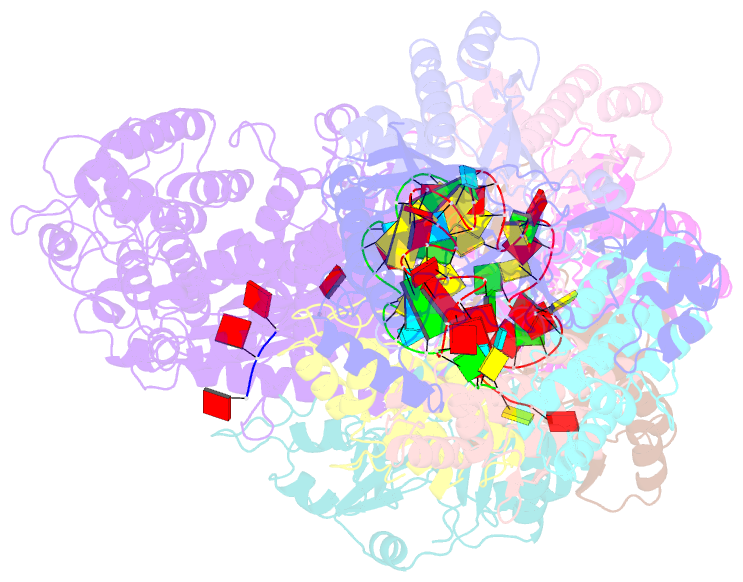
- cryo-EM structure of the active lactococcus lactis csm bound to target in post-cleavage stage
- Reference
- Goswami HN, Ahmadizadeh F, Wang B, Addo-Yobo D, Zhao Y, Whittington AC, He H, Terns MP, Li H (2024): "Molecular basis for cA6 synthesis by a type III-A CRISPR-Cas enzyme and its conversion to cA4 production." Nucleic Acids Res., 52, 10619-10629. doi: 10.1093/nar/gkae603.
- Abstract
- The type III-A (Csm) CRISPR-Cas systems are multi-subunit and multipronged prokaryotic enzymes in guarding the hosts against viral invaders. Beyond cleaving activator RNA transcripts, Csm confers two additional activities: shredding single-stranded DNA and synthesizing cyclic oligoadenylates (cOAs) by the Cas10 subunit. Known Cas10 enzymes exhibit a fascinating diversity in cOA production. Three major forms-cA3, cA4 and cA6have been identified, each with the potential to trigger unique downstream effects. Whereas the mechanism for cOA-dependent activation is well characterized, the molecular basis for synthesizing different cOA isoforms remains unclear. Here, we present structural characterization of a cA6-producing Csm complex during its activation by an activator RNA. Analysis of the captured intermediates of cA6 synthesis suggests a 3'-to-5' nucleotidyl transferring process. Three primary adenine binding sites can be identified along the chain elongation path, including a unique tyrosine-threonine dyad found only in the cA6-producing Cas10. Consistently, disrupting the tyrosine-threonine dyad specifically impaired cA6 production while promoting cA4 production. These findings suggest that Cas10 utilizes a unique enzymatic mechanism for forming the phosphodiester bond and has evolved distinct strategies to regulate the cOA chain length.