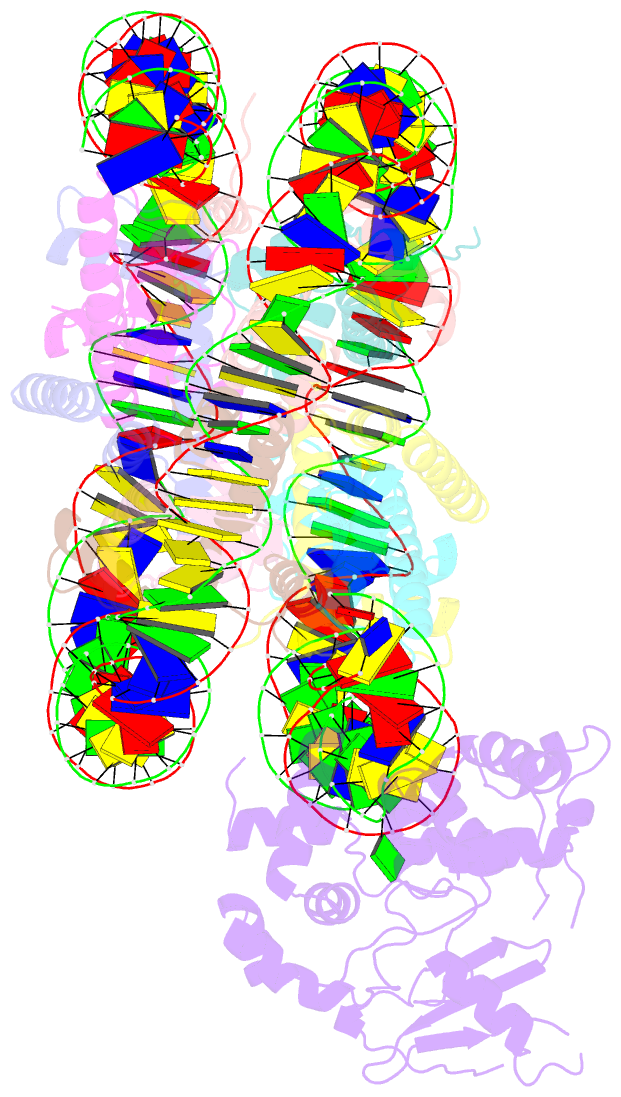
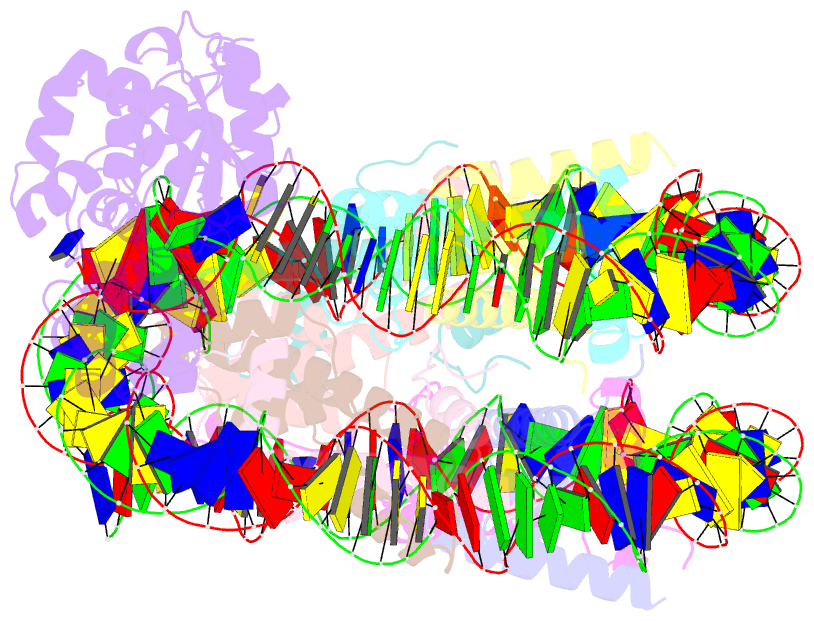
Summary information and primary citation
- PDB-id
-
9eoz;
DSSR-derived features in text and
JSON formats; DNAproDB
- Class
- DNA binding protein
- Method
- cryo-EM (3.1 Å)
- Summary
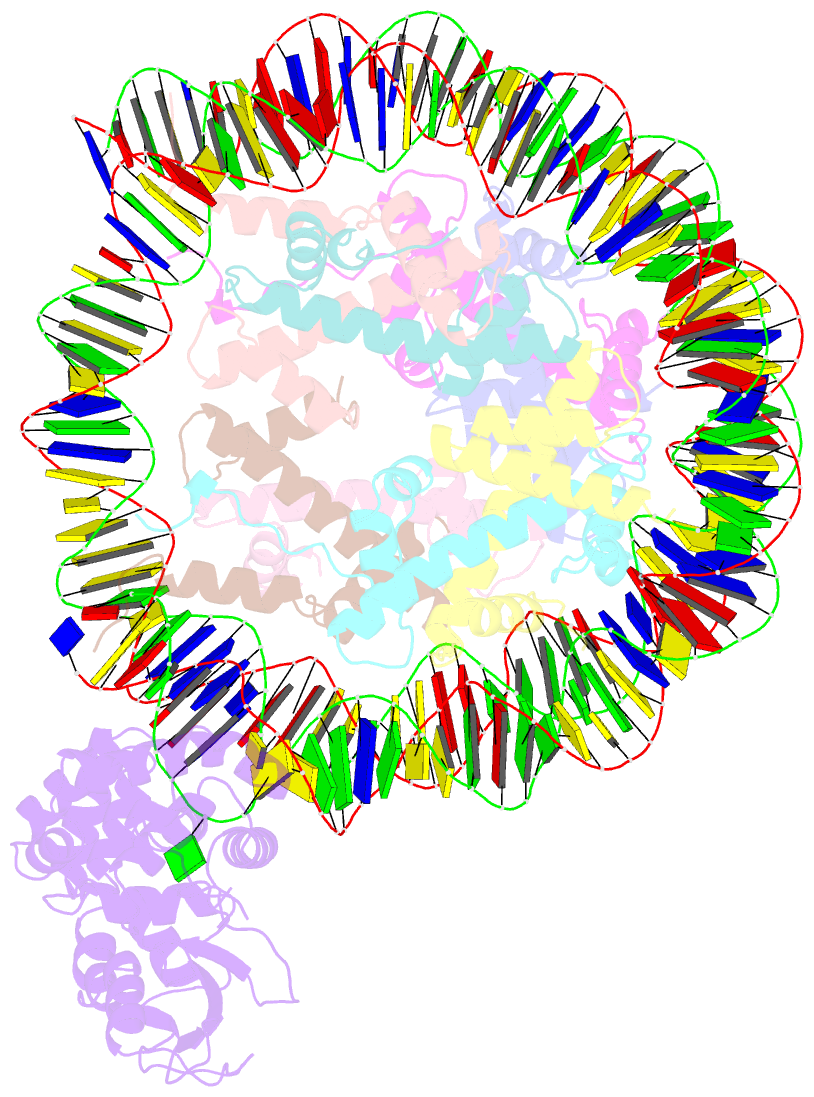
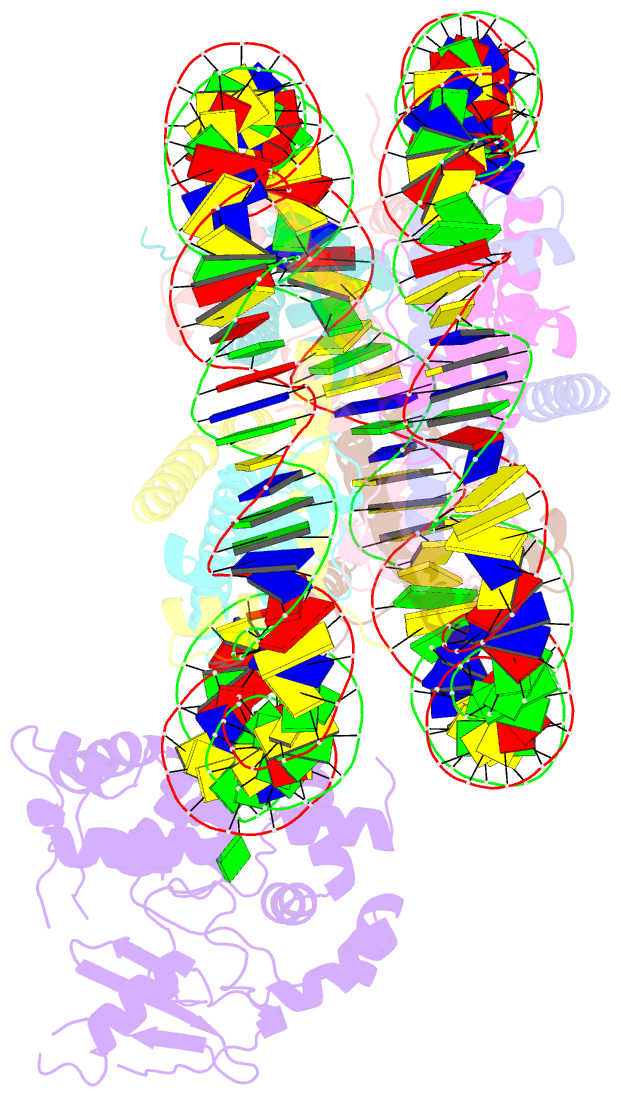
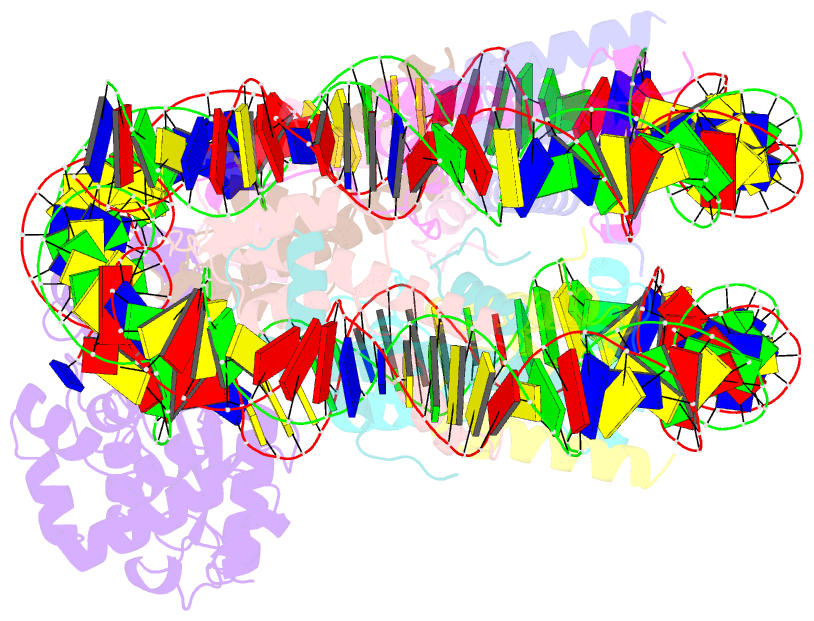
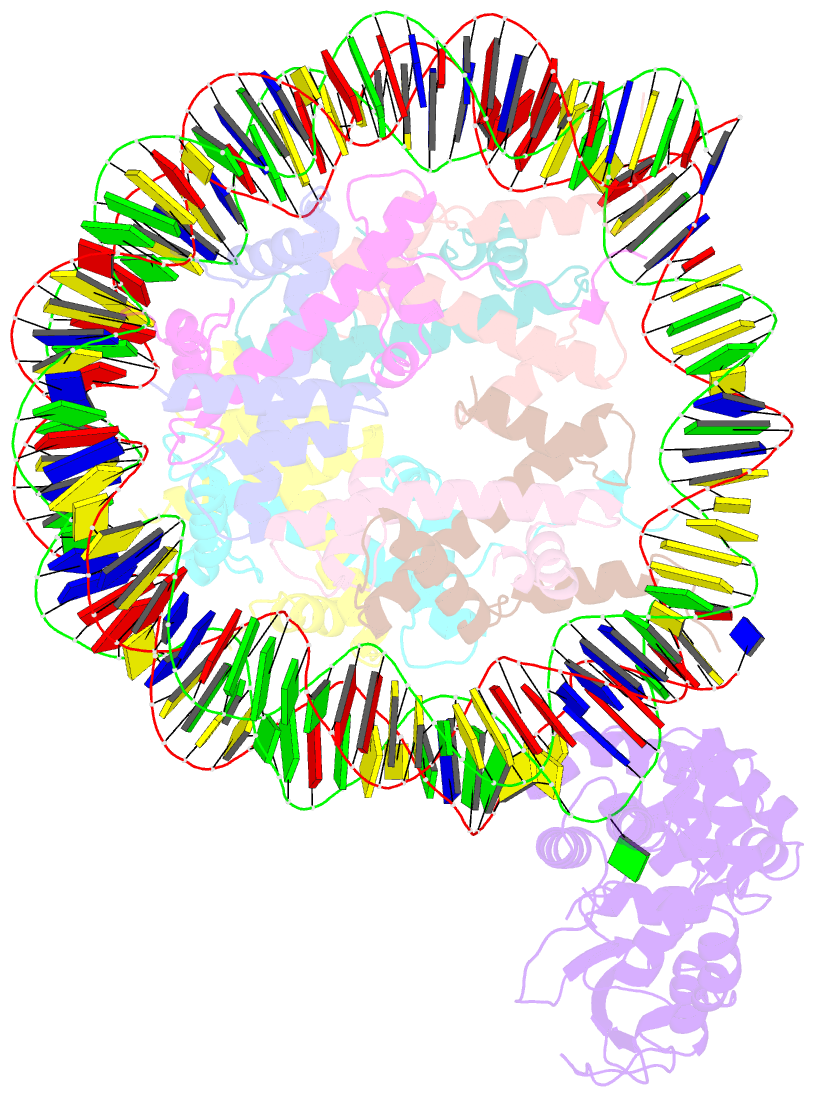
- Human ogg1 bound to a nucleosome core particle with
8-oxodguo lesion at shl6.0
- Reference
-
Ren M, Gut F, Fan Y, Ma J, Shan X, Yikilmazsoy A,
Likhodeeva M, Hopfner KP, Zhou C (2024): "Structural
basis for human OGG1 processing 8-oxodGuo within
nucleosome core particles." Nat Commun,
15, 9407. doi: 10.1038/s41467-024-53811-3.
- Abstract
- Base excision repair (BER) is initialized by DNA
glycosylases, which recognize and flip damaged bases out of
the DNA duplex into the enzymes active site, followed by
cleavage of the glycosidic bond. Recent studies have
revealed that all types of DNA glycosylases repair base
lesions less efficiently within nucleosomes, and their
repair activity is highly depended on the lesion's location
within the nucleosome. To reveal the underlying molecular
mechanism of this phenomenon, we determine the 3.1 Å
cryo-EM structure of human 8-oxoguanine-DNA glycosylase 1
(hOGG1) bound to a nucleosome core particle (NCP)
containing a common oxidative base lesion,
8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxodGuo). Our
structural analysis shows that hOGG1 can recognize and flip
8-oxodGuo even within NCPs; however, the interaction
between 8-oxodGuo and hOGG1 in a NCP context is weaker than
in free DNA due to competition for nucleosomal DNA by the
histones. Binding of OGG1 and the flipping of 8-oxodGuo by
hOGG1 leads to a partial detachment of DNA from the histone
core and a ratchet-like inward movement of nucleosomal DNA.
Our findings provide insights into how the dynamic
structure of nucleosomes modulate the activity of repair
enzymes within chromatin.