Summary information and primary citation
- PDB-id
- 9ffb; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- cryo-EM (3.59 Å)
- Summary
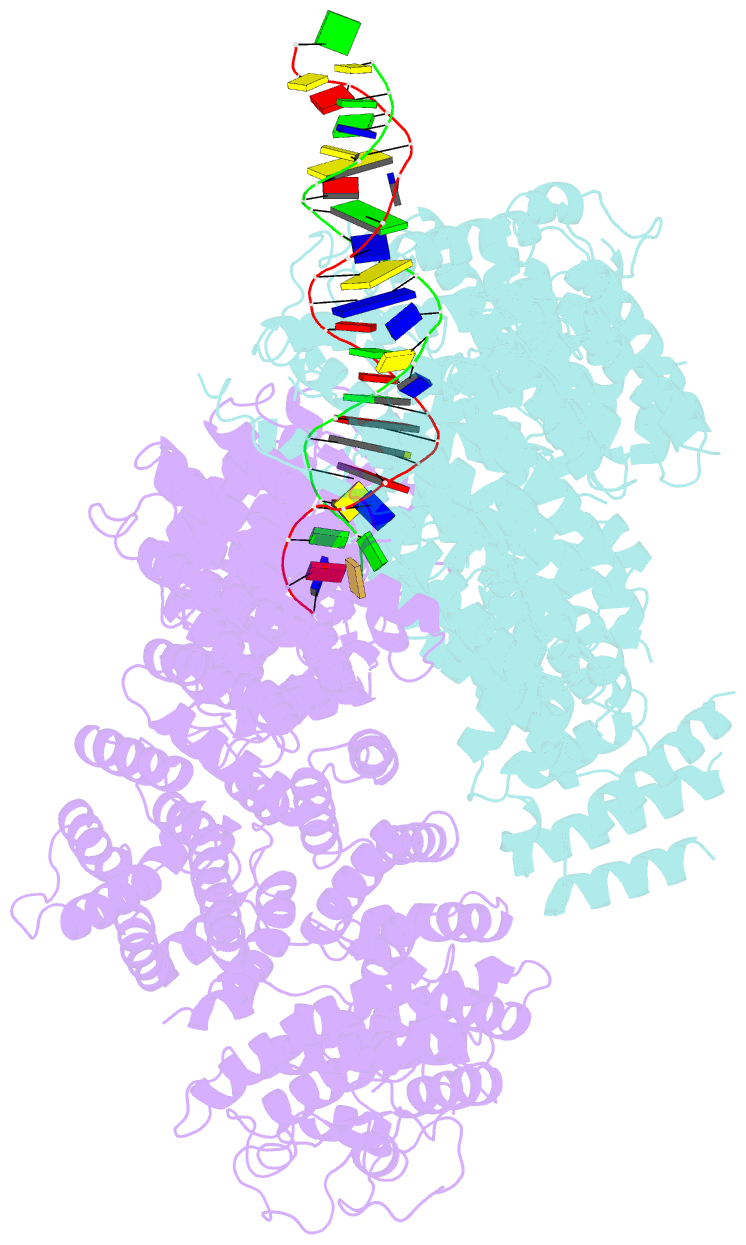
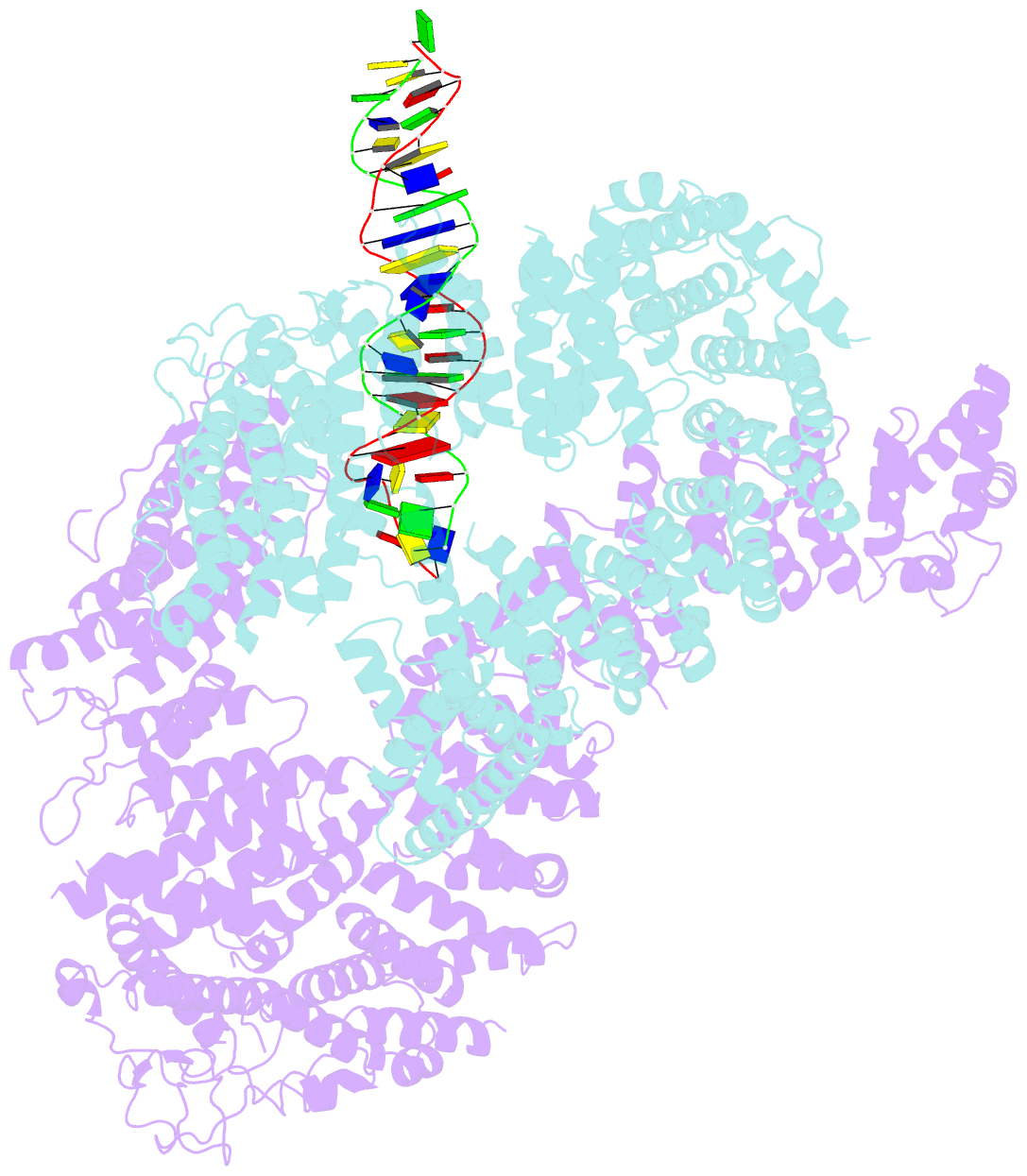
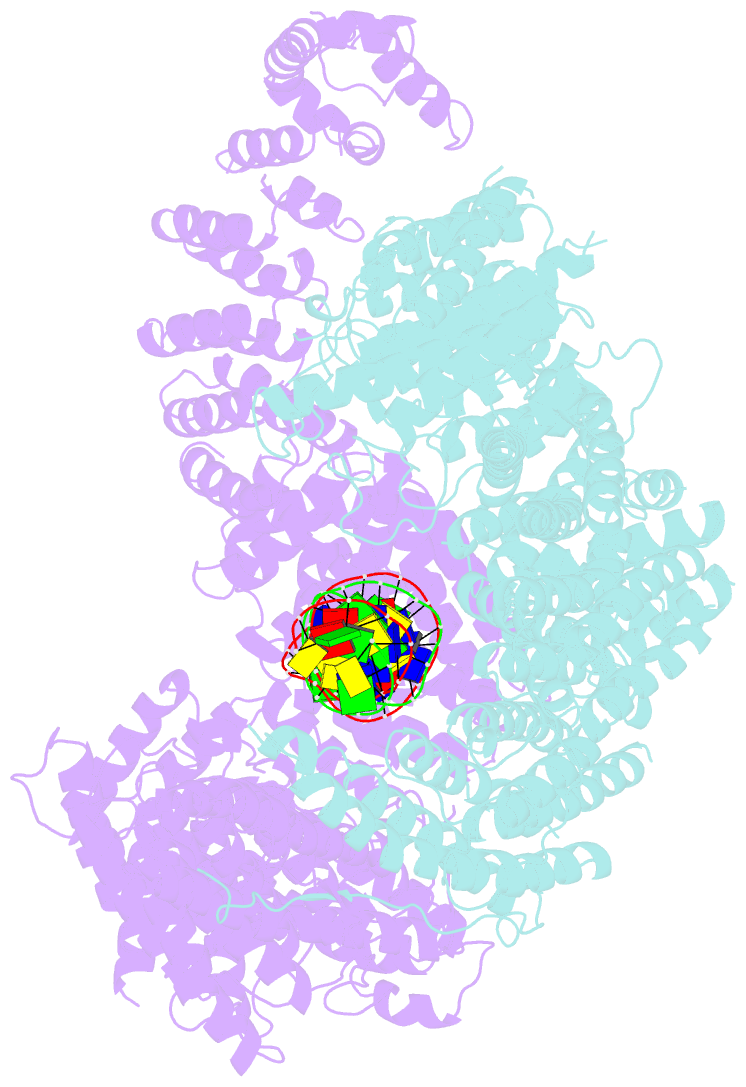
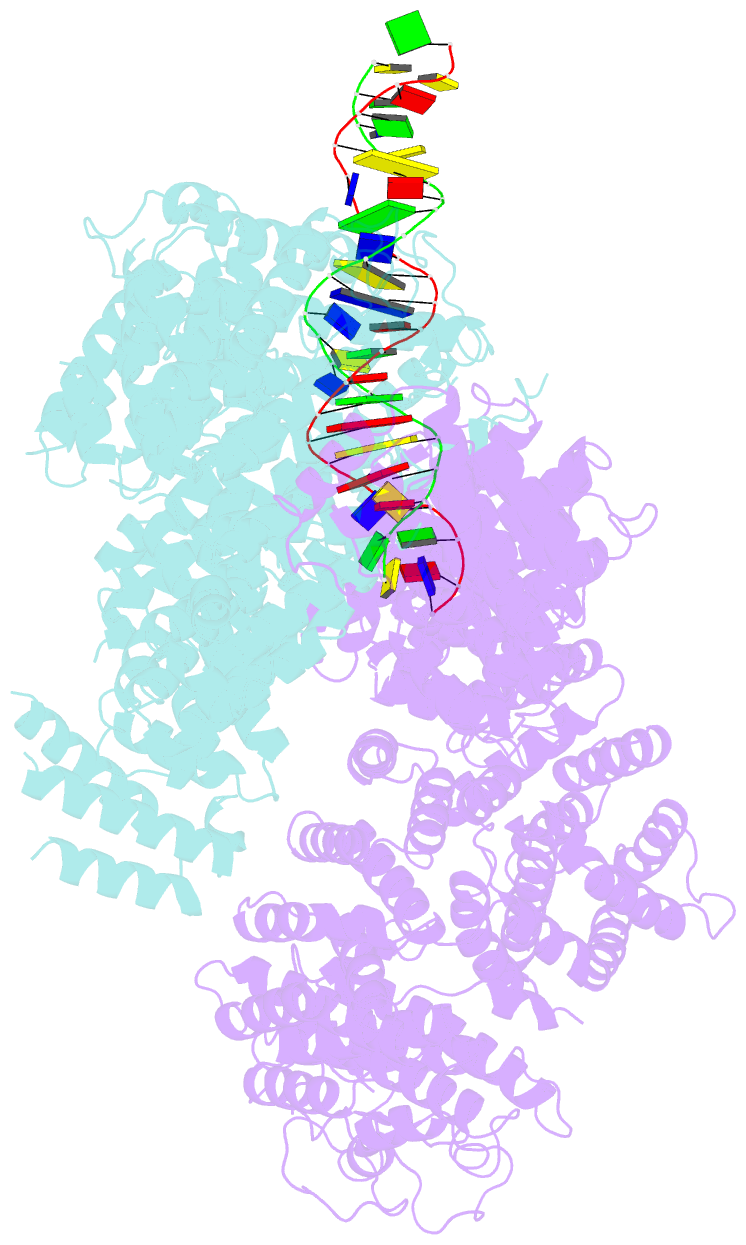
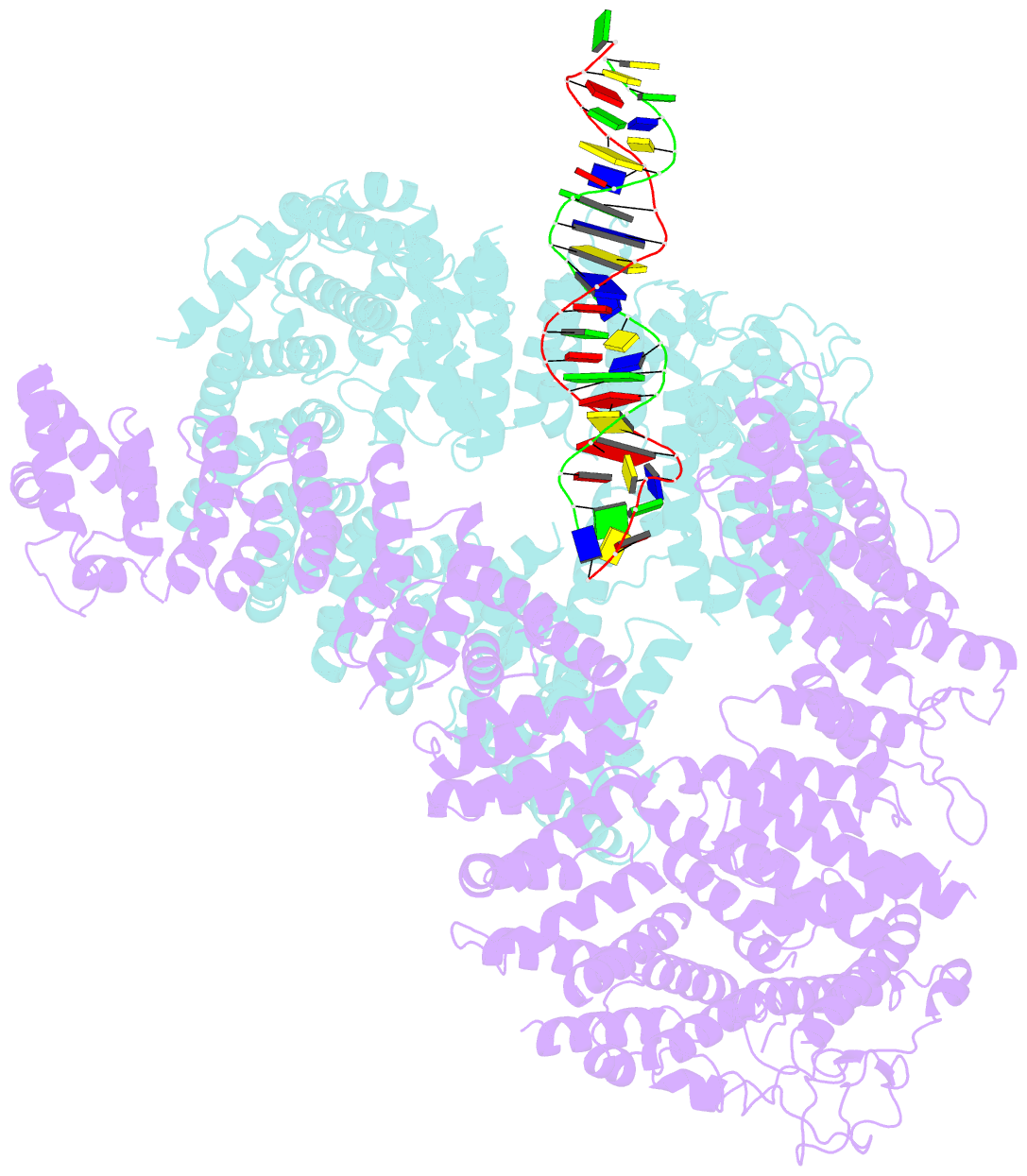
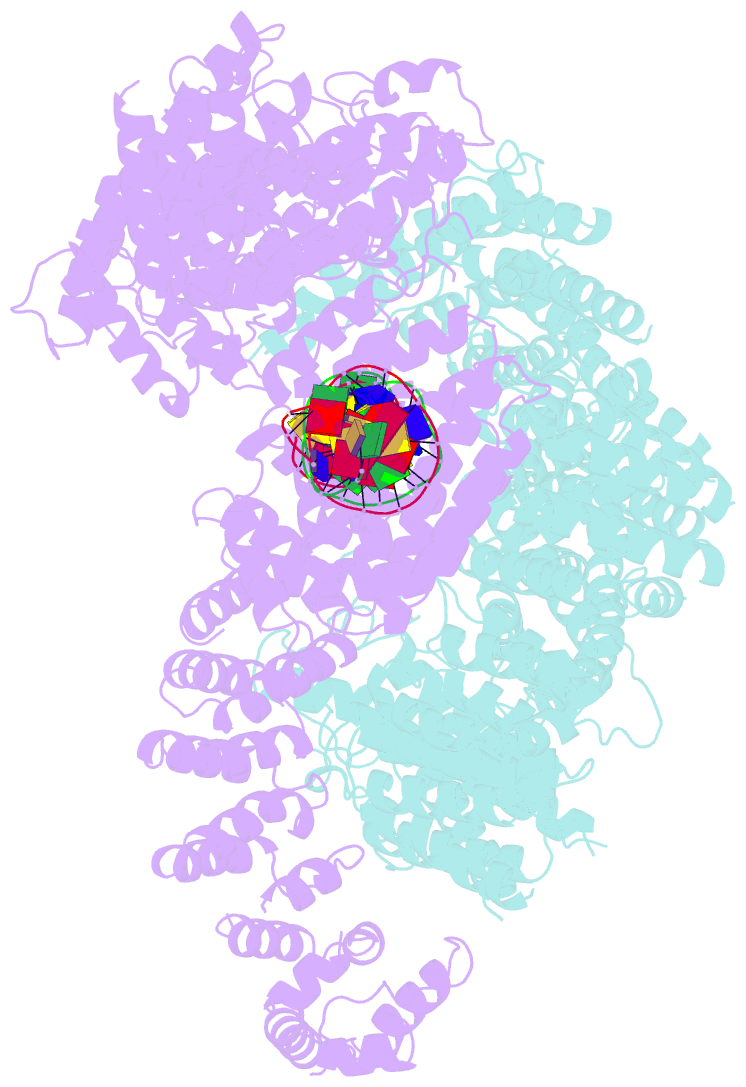
- Ss-dsDNA-fancd2-fanci complex
- Reference
- Alcon P, Kaczmarczyk AP, Ray KK, Liolios T, Guilbaud G, Sijacki T, Shen Y, McLaughlin SH, Sale JE, Knipscheer P, Rueda DS, Passmore LA (2024): "FANCD2-FANCI surveys DNA and recognizes double- to single-stranded junctions." Nature, 632, 1165-1173. doi: 10.1038/s41586-024-07770-w.
- Abstract
- DNA crosslinks block DNA replication and are repaired by the Fanconi anaemia pathway. The FANCD2-FANCI (D2-I) protein complex is central to this process as it initiates repair by coordinating DNA incisions around the lesion1. However, D2-I is also known to have a more general role in DNA repair and in protecting stalled replication forks from unscheduled degradation2-4. At present, it is unclear how DNA crosslinks are recognized and how D2-I functions in replication fork protection. Here, using single-molecule imaging, we show that D2-I is a sliding clamp that binds to and diffuses on double-stranded DNA. Notably, sliding D2-I stalls on encountering single-stranded-double-stranded (ss-ds) DNA junctions, structures that are generated when replication forks stall at DNA lesions5. Using cryogenic electron microscopy, we determined structures of D2-I on DNA that show that stalled D2-I makes specific interactions with the ss-dsDNA junction that are distinct from those made by sliding D2-I. Thus, D2-I surveys dsDNA and, when it reaches an ssDNA gap, it specifically clamps onto ss-dsDNA junctions. Because ss-dsDNA junctions are found at stalled replication forks, D2-I can identify sites of DNA damage. Therefore, our data provide a unified molecular mechanism that reconciles the roles of D2-I in the recognition and protection of stalled replication forks in several DNA repair pathways.